Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Solubility Rules for Salts
Solubility Rules for Salts | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Solubility Rules
- Salts are prepared using different methods based on their solubility, highlighting the importance of understanding salt solubility.
- Salts are prepared using different methods based on their solubility, highlighting the importance of understanding salt solubility.
Solubility of the common salts
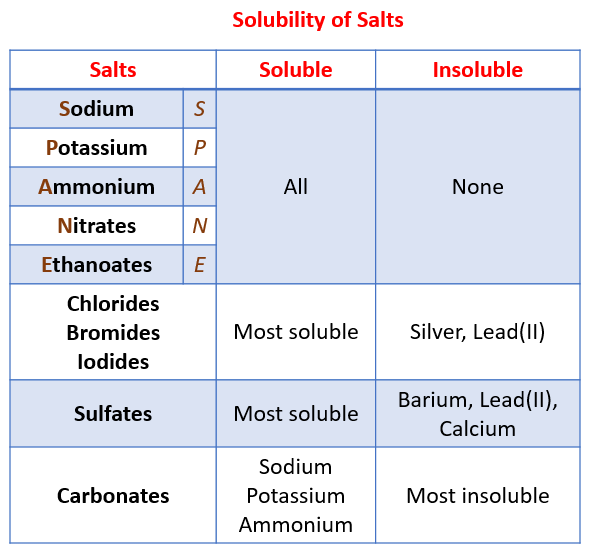
Question for Solubility Rules for SaltsTry yourself: Which of the following salts is most likely to be soluble in water?View Solution
The document Solubility Rules for Salts | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Solubility Rules for Salts - Chemistry for GCSE/IGCSE - Class 10
| 1. What are solubility rules for salts? |  |
Ans. Solubility rules for salts are guidelines that help predict whether a salt will dissolve in water or not. These rules are based on the principle that like dissolves like, meaning salts with similar properties will dissolve in water.
| 2. How can solubility rules be used to determine if a salt will dissolve in water? |  |
Ans. By referring to solubility rules, one can determine if a salt will dissolve in water by looking at the ions present in the salt. If the combination of ions in the salt follows the rules of solubility, then the salt will dissolve in water.
| 3. What are some common solubility rules for salts? |  |
Ans. Some common solubility rules for salts include that all salts of sodium, potassium, and ammonium are soluble, most nitrates are soluble, most chlorides are soluble except for silver chloride and lead chloride, and most sulfates are soluble except for those of calcium, strontium, and barium.
| 4. Why is it important to understand solubility rules for salts? |  |
Ans. Understanding solubility rules for salts is important as it helps predict whether a salt will dissolve in water or not, which is crucial in various chemical reactions and processes. It also aids in determining the products of reactions and their solubility.
| 5. Can solubility rules be applied to all salts? |  |
Ans. While solubility rules provide a general guideline for predicting the solubility of salts, there are exceptions to these rules. Some salts may not follow the typical solubility patterns due to unique properties or interactions between ions.
Related Searches
















