Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Hydrous and Anhydrous Substance
Hydrous and Anhydrous Substance | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Hydrated & Anhydrous Salts
- When salts are being prepared, some water can be retained within the structure of the salt during the crystallisation process
- This affects the crystal's shape and color
- Salts that contain water within their structure are called hydrated salts
- Anhydrous salts are those that contain no water in their structure
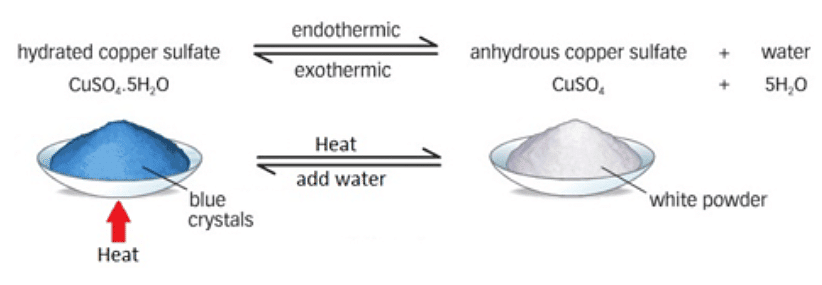
- For instance, consider copper(II) sulfate, which crystallizes to form hydrated copper(II) sulfate, appearing blue
- Upon heating, water is expelled from its structure, resulting in anhydrous copper(II) sulfate, which is white
- The process of removing water from the hydrated salt to form the anhydrous salt is known as dehydration
- Interestingly, the reaction can be reversed by introducing water to anhydrous copper(II) sulfate:
hydrated copper(II) sulfate ↔ anhydrous copper(II) sulfate + water
Question for Hydrous and Anhydrous SubstanceTry yourself: What is the difference between hydrated and anhydrous salts?View Solution
Water of Crystallisation
- Water molecules present in certain salts during crystallization are termed as water of crystallisation.
- A hydrated compound refers to a substance containing water of crystallisation within its structure.
- When representing the chemical formula of hydrated compounds, the water of crystallisation is denoted by a dot. For instance, hydrated copper(II) sulfate is written as CuSO4 ∙ 5H2O.
- The formula indicates the quantity of water molecules present in one mole of the hydrated salt. For example, hydrated copper(II) sulfate, CuSO4 ∙ 5H2O, contains 5 moles of water in 1 mole of the hydrated salt.
- An anhydrous compound is a substance that lacks water of crystallisation. An example is anhydrous copper(II) sulfate, CuSO4.
- The conversion between anhydrous and hydrated compounds is reversible. Heating the hydrated salt leads to the formation of the anhydrous salt, and vice versa.
Hydrated Compounds
- Hydrated copper(II) sulfate, CuSO4 ∙ 5H2O, contains 5 moles of water in 1 mole of the hydrated salt.
- The formula illustrates the number of moles of water present within one mole of the hydrated salt.
Anhydrous Compounds
- An anhydrous compound, such as anhydrous copper(II) sulfate (CuSO4) or anhydrous cobalt(II) chloride (CoCl2), does not contain water of crystallization.
Reversibility of Conversion
The conversion between anhydrous and hydrated compounds is reversible through heating. For instance:
- Anhydrous to hydrated salt: CuSO4 + 5H2O → CuSO4 ∙ 5H2O
- Hydrated to anhydrous salt (by heating): CuSO4 ∙ 5H2O → CuSO4 + 5H2O
Diagram showing the dehydration of hydrated copper II) sulfate:
The document Hydrous and Anhydrous Substance | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Hydrous and Anhydrous Substance - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the difference between hydrated and anhydrous salts? |  |
Ans. Hydrated salts contain water molecules within their crystal structure, known as water of crystallization, while anhydrous salts do not contain any water molecules.
| 2. How does the presence of water of crystallization affect the properties of hydrated salts? |  |
Ans. The water of crystallization can affect the color, solubility, and stability of hydrated salts. It can also impact the crystal structure and physical properties of the salts.
| 3. What is the significance of distinguishing between hydrous and anhydrous substances in chemistry? |  |
Ans. Understanding whether a substance is hydrous or anhydrous is crucial for accurately determining its composition, properties, and behavior in chemical reactions. It also helps in predicting the stability and reactivity of the substance.
| 4. How can one determine the water of crystallization in a hydrated salt experimentally? |  |
Ans. The water of crystallization in a hydrated salt can be determined experimentally by heating the salt to drive off the water molecules and measuring the weight loss. The difference in weight before and after heating corresponds to the water of crystallization.
| 5. Can hydrated salts be converted into anhydrous salts, and vice versa? |  |
Ans. Yes, hydrated salts can be converted into anhydrous salts by heating them to remove the water of crystallization. Similarly, anhydrous salts can be converted into hydrated salts by adding water to them to allow for the formation of crystals with water molecules.
Related Searches















