Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Periodic Trends
Periodic Trends | Chemistry for GCSE/IGCSE - Class 10 PDF Download
| Table of contents |

|
| The Metallic Character of Elements |

|
| Periodic Trends & Electronic Configuration |

|
| Predicting Properties |

|
| Identifying Trends |

|
The Metallic Character of Elements
- As you progress across a Period on the Periodic Table from left to right, the metallic character of elements diminishes, while it increases when moving down a Group.
- This trend arises because atoms have a greater tendency to accept electrons to achieve a full valence shell rather than losing them to attain a full outer shell.
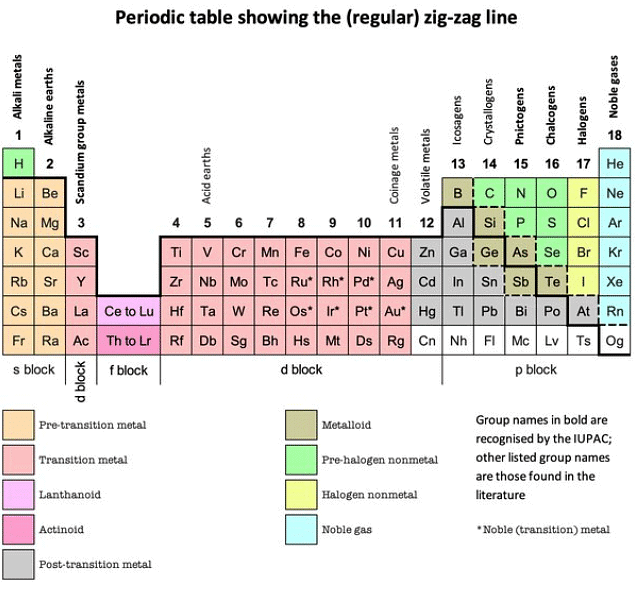
- Metals are situated on the left side of the Periodic Table, whereas non-metals occupy the right side.
- Positioned between metals and non-metals are elements that exhibit characteristics of both, known as metalloids or semi-metals.
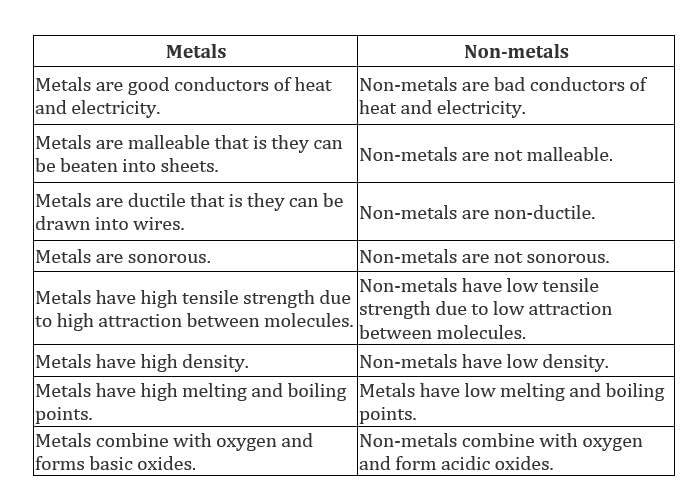
Properties of metals and non-metals
Periodic Trends & Electronic Configuration
- The arrangement of electrons into shells for an atom is known as the electronic configuration. For instance, the electronic configuration of carbon is 2,4.
- There exists a correlation between the electronic configuration of elements and their positions on the Periodic Table.
- The number of notations in the electronic configuration indicates the number of occupied electron shells of the atom, which in turn reveals the period.
- The last notation in the electronic configuration signifies the number of outer electrons the atom possesses, indicating the group number.
Example: Electronic configuration of chlorine:
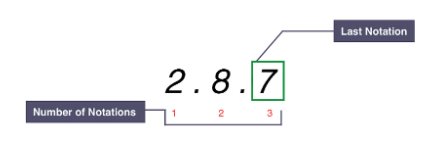
The electronic configuration of chlorine:
- The red numbers at the bottom indicate 3 shells of electrons for a chlorine atom.
- The final notation of 7 signifies 7 outer electrons, placing chlorine in Group VII.
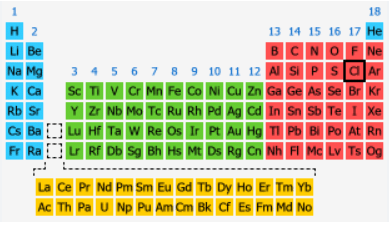
The Position of Chlorine on the Periodic Table:
Elements in the Same Group
- Elements in the same group exhibit similar chemical properties due to their outermost electrons interacting.
- Similarity in properties arises from having the same number of electrons in the outer shell.
- Lithium and sodium, both in Group I, can form compounds by donating electrons to elements in Group VII.
- Down a group, each subsequent element adds a full shell of electrons.
- Lithium's electronic configuration: 2,1
- Sodium's electronic configuration: 2,8,1
- Potassium's electronic configuration: 2,8,8,1
Question for Periodic TrendsTry yourself: Which trend explains the change in metallic character of elements across a period on the Periodic Table?View Solution
Predicting Properties
- The arrangement of elements on the Periodic Table reflects patterns in both their chemical behavior and physical properties.
- These trends manifest both vertically down groups and horizontally across periods.
- Utilizing the Periodic Table enables the prediction of various properties like boiling point, melting point, density, and reactivity.
- Some notable trends include the rapid reactivity of Group I elements with water, the inertness of noble gases, and the higher density of transition elements compared to Group I elements.
- Reactivity diminishes down Group VII, while melting point decreases down Group I.
- Consequently, the Periodic Table serves as a tool for anticipating the behavior of specific elements.
Identifying Trends
- By analyzing information about elements, we can recognize patterns in their properties.
- For instance, this analysis helps us determine how the reactivity of Group I metals changes.
- Below is a summary of the reactions of the initial three Group I elements with water.
- Observations of Lithium, Sodium, and Potassium with Water:
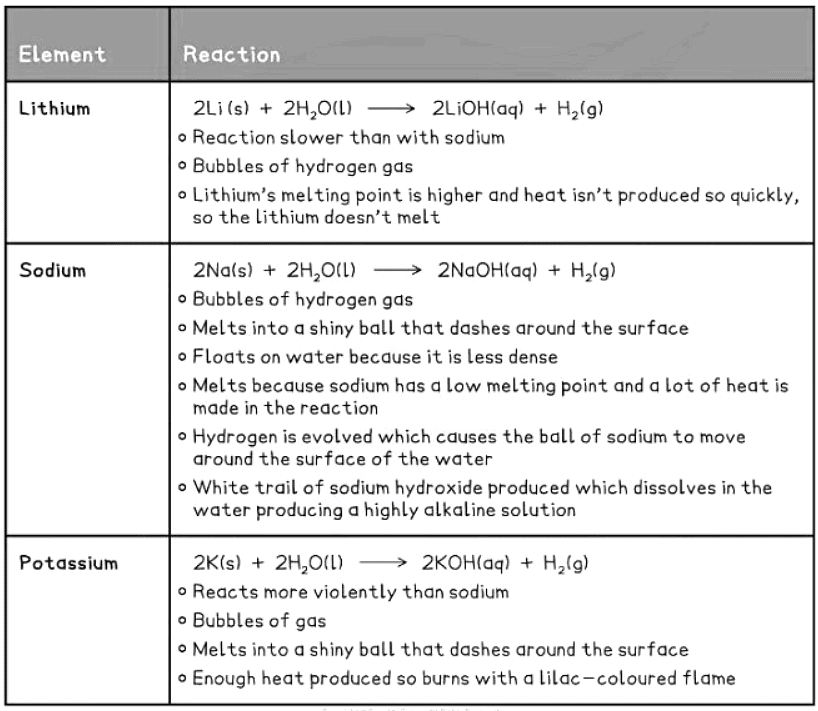
- The observations indicate that the reactivity of Group I metals increases as we move down the group.
- This information allows us to anticipate the trend for rubidium, caesium, and francium as we progress down Group I.
- As alkali metals become more reactive down the group, rubidium, caesium, and francium exhibit more vigorous reactions with air and water compared to lithium, sodium, and potassium.
- Among these metals, lithium is the least reactive at the top, while francium is the most reactive at the bottom.
- Due to its rarity and radioactivity, confirming predictions related to francium can be challenging.
Table to Show the Predicted Reaction of other Group I Elements with Water
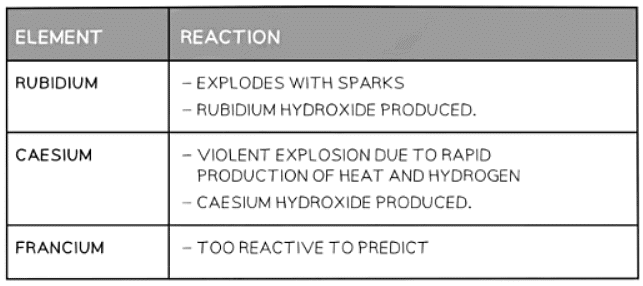
Question for Periodic TrendsTry yourself: Which Group I element is predicted to have the most vigorous reaction with water?View Solution
The document Periodic Trends | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Periodic Trends - Chemistry for GCSE/IGCSE - Class 10
| 1. What is metallic character in the periodic table? |  |
Ans. Metallic character refers to the ability of an element to exhibit properties of a metal, such as conducting electricity and heat. Elements on the left side of the periodic table are more metallic in character.
| 2. How does electronic configuration affect metallic character? |  |
Ans. Elements with more electrons in their outer shell tend to exhibit more metallic character as they can easily lose electrons to form positive ions, which is a characteristic of metals.
| 3. What are the trends in metallic character across a period and down a group in the periodic table? |  |
Ans. Metallic character decreases across a period from left to right due to increasing effective nuclear charge, while it increases down a group due to the increase in the number of electron shells.
| 4. How can metallic character be predicted based on the electronic configuration of an element? |  |
Ans. Elements with one or two electrons in their outer shell are more likely to exhibit metallic character, while elements with a full outer shell or close to it tend to be nonmetals.
| 5. Can metallic character be used to predict other properties of elements? |  |
Ans. Yes, metallic character is closely related to other properties such as reactivity, melting and boiling points, and conductivity. Elements with high metallic character tend to have higher reactivity and conductivity.
Related Searches














