Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Transition Elements
Transition Elements | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Transition Elements
General properties of the transition elements
- They are very hard and strong metals and are good conductors of heat and electricity
- They have very high melting points and are highly dense metals
- For instance, titanium has a melting point of 1,688°C, significantly higher than potassium in Group I which melts at only 63.5°C, just a bit warmer than a cup of hot chocolate!
- Transition elements are known for forming colored compounds and often exhibit more than one oxidation state. For example, iron can readily form compounds of both Fe2 and Fe3.
- These colored compounds contribute to the pigments found in various paints as well as the hues of gemstones and rocks.
- Transition elements, whether in their elemental form or as compounds, are frequently employed as catalysts to enhance reaction rates in industrial processes. Catalysts like platinum or rhodium, which are transition elements, are utilized in catalytic converters in vehicles to reduce the levels of nitrous oxides and carbon monoxide emissions.
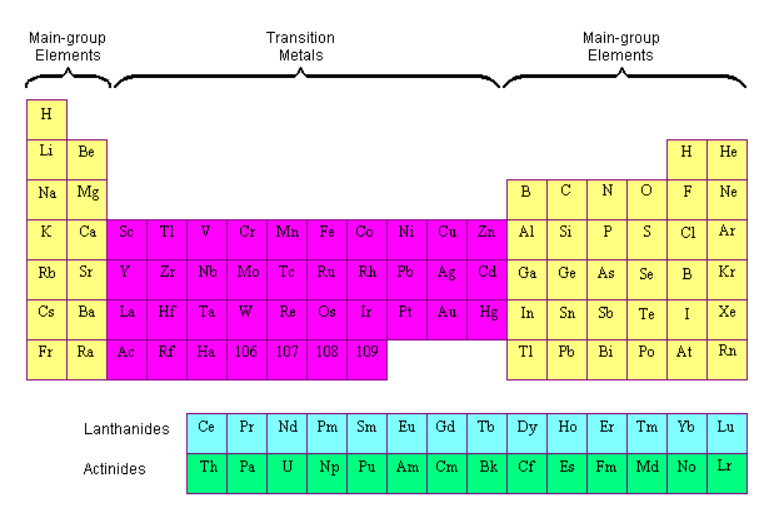
- The transition elements on the Periodic Table:
Question for Transition ElementsTry yourself: Which property of transition elements allows them to be used as catalysts in industrial processes?View Solution
Transition Elements Oxidation Numbers
- The transition elements exhibit multiple oxidation numbers, enabled by their capacity to shed varying numbers of electrons contingent upon their chemical surroundings.
- For instance: iron either:
- Iron can either lose two electrons to generate Fe2+, representing an oxidation state of +2, or shed three electrons to form Fe3+, signifying an oxidation state of +3.
- Compounds containing transition elements in diverse oxidation states exhibit distinct properties and colors.
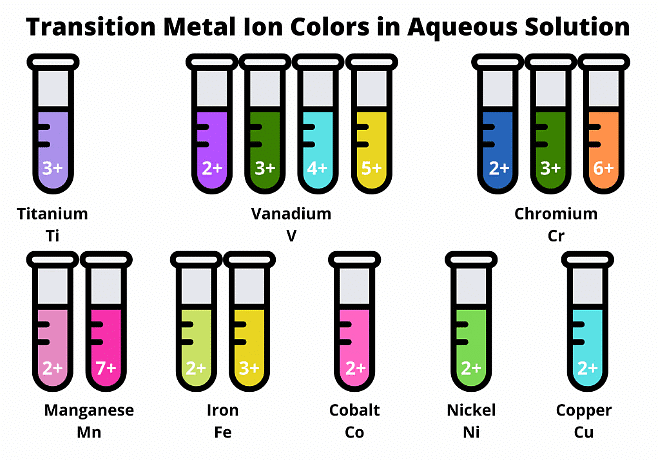
Uses of the transition elements
- Transition elements serve widely as catalysts owing to their capacity to transition between various oxidation states.
- This flexibility enables them to create complexes with reagents that readily donate and accept electrons in chemical reactions.
- Their applications extend to medicine and surgical procedures, including limb and joint replacements where titanium's high biocompatibility facilitates bone bonding.
- Additionally, they contribute to the production of colored compounds for dyes, paints, and stained glass jewelry.
Question for Transition ElementsTry yourself: Which oxidation states can iron exhibit?View Solution
The document Transition Elements | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Transition Elements - Chemistry for GCSE/IGCSE - Class 10
| 1. What are transition elements? |  |
Ans. Transition elements are elements in the d-block of the periodic table that have partially filled d orbitals in their atomic or ionic states.
| 2. How do transition elements exhibit variable oxidation numbers? |  |
Ans. Transition elements can exhibit variable oxidation numbers due to the availability of electrons in the d orbitals, allowing them to lose or gain electrons in different ways.
| 3. Why are transition elements important in chemistry? |  |
Ans. Transition elements play a crucial role in catalysis, industrial processes, and biological systems due to their unique electronic configurations and chemical properties.
| 4. Can transition elements form complex ions? |  |
Ans. Yes, transition elements can form complex ions by bonding with other molecules or ions through coordinate covalent bonds, creating stable and colorful compounds.
| 5. How do transition elements contribute to the color of transition metal complexes? |  |
Ans. Transition elements contribute to the color of transition metal complexes through the absorption and reflection of specific wavelengths of light, which result from the d-d electronic transitions within the complex ions.
Related Searches















