Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Reactivity Series
Reactivity Series | Chemistry for GCSE/IGCSE - Class 10 PDF Download
| Table of contents |

|
| What is the reactivity series of metals? |

|
| How to remember the reactivity series |

|
| Reactions of Metals |

|
| The Order of Reactivity of Metals |

|
What is the reactivity series of metals?
- The study of metal chemistry involves analyzing their reactions with water and acids.
- Observing these reactions allows for the creation of a reactivity series that orders metals based on their reactivity levels.
- This series is useful for arranging metals in terms of reactivity by observing their reactions with water and acids.
- Hydrogen and carbon, despite being non-metals, are part of the reactivity series due to their role in extracting metals from their oxides.
- The reactivity series includes non-metals like hydrogen and carbon, essential for metal extraction from oxides.
Reactivity Series of Metals
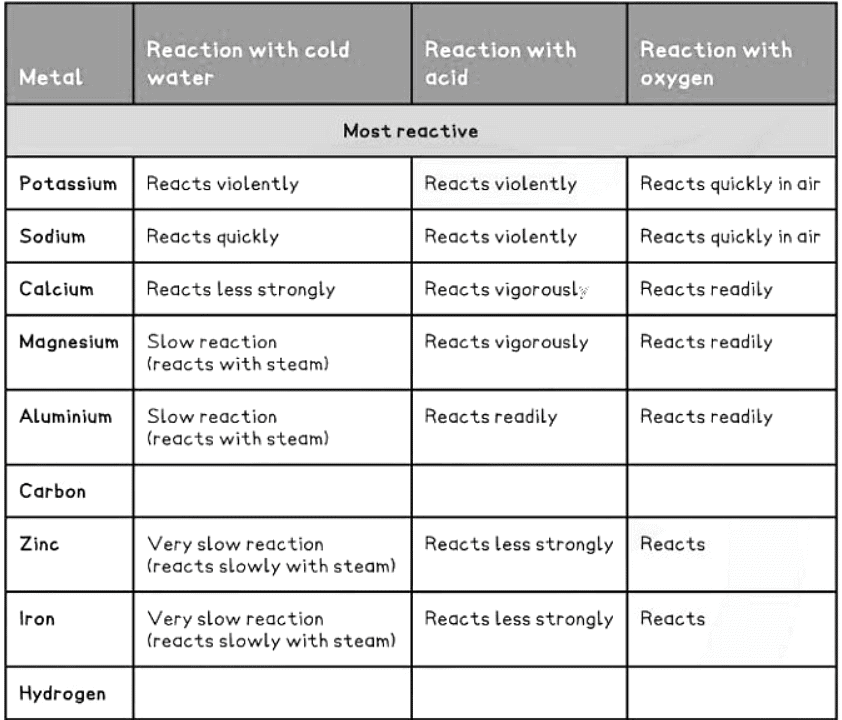
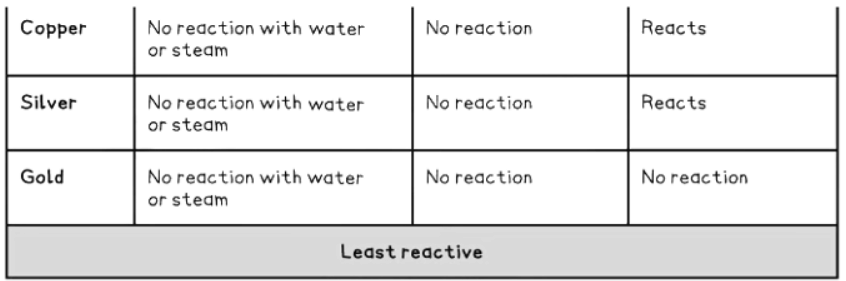
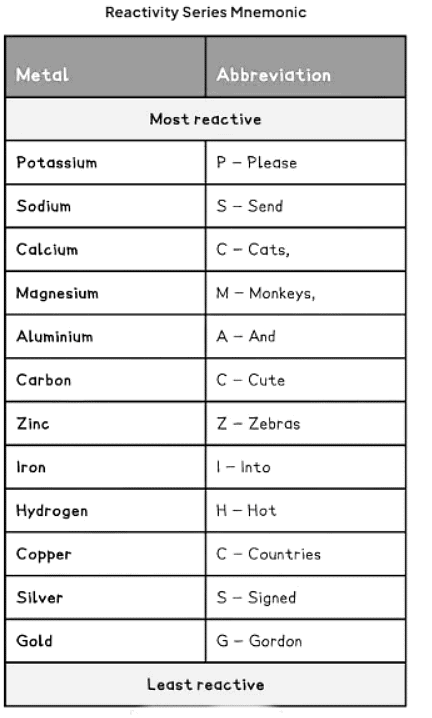
How to remember the reactivity series
- Observations from the table above allow us to deduce the reactivity series.
- We can memorize the order of the reactivity series with the mnemonic: "Please send cats, monkeys and cute zebras into hot countries signed Gordon."
Question for Reactivity SeriesTry yourself: Which of the following is the correct order of metals in the reactivity series?View Solution
Reactions of Metals
Reaction with cold water
- The more reactive metals will react with cold water to produce a metal hydroxide along with hydrogen gas.
- Examples include potassium, sodium, and calcium, as they are highly reactive and undergo reactions with cold water.
Metal + Water → Metal Hydroxide + Hydrogen - Calcium and Potassium Reactions:
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
K (s) + H2O (l) → KOH (aq) + H2 (g)
Reacting with Steam
Metals below calcium in the reactivity series, like magnesium, react with steam to form a metal oxide and hydrogen gas:
Mg (s) + H2O (g) → MgO (s) + H2 (g)
Reaction with dilute acids
- Metal Reactivity with Acids: Only metals above hydrogen in the reactivity series will react with dilute acids.
- Unreactive Metals: Metals like gold, silver, and copper, which are below hydrogen, do not react with acids.
- Reactivity Levels: The more reactive the metal, the more vigorous the reaction.
- High Reactivity Metals: Metals high in the reactivity series, such as potassium and sodium, react explosively with acids.
- Acid-Metal Reactions: When acids react with metals, they produce a salt and hydrogen gas: metal + acid → salt + hydrogen
- Some examples of metal-acid reactions and their equations are given below:
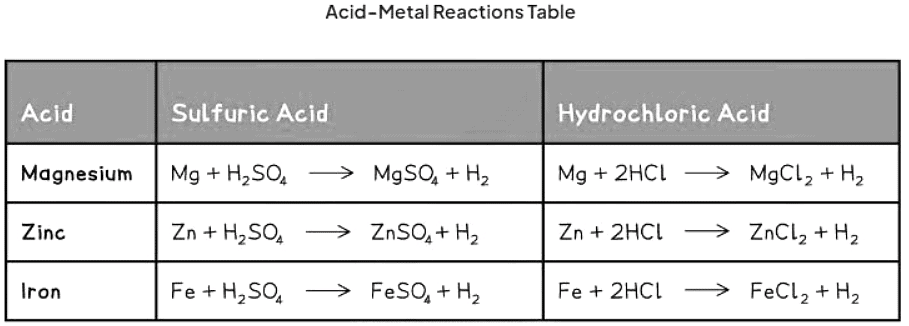
Reaction with oxygen
- Reactive metals like alkali metals easily react with oxygen.
- Silver, copper, and iron can also react with oxygen, albeit at a slower rate.
- Upon reacting with oxygen, metals form metal oxides. For instance, copper reacts as follows:
metal + oxygen → metal oxide
2Cu (s) + O2 (g) → 2CuO (s) - Gold, however, does not react with oxygen.
The Order of Reactivity of Metals
- The reactivity series of metals can be determined through experimental observations of their reactions with water, acids, and oxygen.
- A metal's position in the reactivity series correlates with the intensity of its reaction - more vigorous reactions signify higher positions in the series.
- For highly reactive metals, their reactivity order can be established by observing their reactions with water.
- Less reactive metals either react slowly or not at all with water, necessitating the observation of their reactions with dilute acid to determine their reactivity order.
- Changes in temperature during a reaction can also indicate the order of reactivity; a significant temperature change suggests higher reactivity.
Examples:
- Sodium (Na) and Water: Sodium reacts vigorously with water, producing hydrogen gas and a strong alkaline solution of sodium hydroxide. This reaction indicates sodium's high reactivity.
- Gold (Au) and Water: Gold, being a noble metal, does not react with water under normal conditions, showcasing its low reactivity.
Question for Reactivity SeriesTry yourself: Which of the following metals reacts vigorously with water?View Solution
The document Reactivity Series | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Reactivity Series - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the reactivity series of metals? |  |
Ans. The reactivity series of metals is a list that ranks metals in order of their reactivity with other substances.
| 2. How can one remember the reactivity series of metals? |  |
Ans. One way to remember the reactivity series is through mnemonics such as "Please Stop Calling Me A Cute Zebra, I Think She's Hot!" where the first letter of each word corresponds to the order of reactivity of metals (Potassium, Sodium, Calcium, Magnesium, Aluminum, Carbon, Zinc, Iron, Tin, Lead, Hydrogen).
| 3. What are some reactions of metals based on their reactivity series? |  |
Ans. Metals higher in the reactivity series will react more vigorously with acids and water, while metals lower in the series are less reactive and may not react with acids or water at all.
| 4. How can the order of reactivity of metals be useful in practical applications? |  |
Ans. The reactivity series helps predict the behavior of metals in various chemical reactions, such as determining which metal will displace another from a compound or which metal will react most readily with a certain substance.
| 5. Why is it important to understand the reactivity series of metals? |  |
Ans. Understanding the reactivity series of metals is crucial in chemistry as it helps explain and predict the outcomes of various chemical reactions involving metals. It also assists in choosing the right metal for specific applications based on its reactivity.
Related Searches




















