Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Galvanisation
Galvanisation | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Galvanising & Sacrificial Protection
- Iron can be prevented from rusting using the reactivity series
Sacrificial Protection
- A more reactive metal can be attached to a less reactive metal
- The more reactive metal will oxidize and corrode first, protecting the less reactive metal
- Example: Using zinc bars on steel ships
- Diagram illustrating the use of zinc bars on steel ships for sacrificial protection:
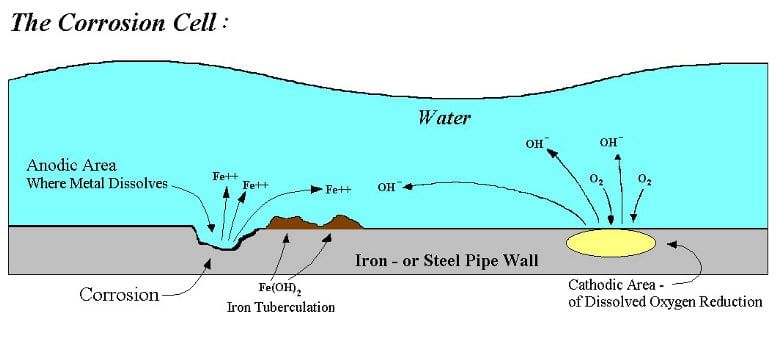
- Zinc is more reactive than iron, making it more prone to losing electrons and getting oxidized easily:
Zn → Zn2+ + 2e- - Iron, being less reactive, does not lose electrons easily and is not readily oxidized. In protective scenarios, zinc is utilized to safeguard steel: Iron: The iron is less reactive, hence not easily oxidized; zinc is used to protect the steel
- Sacrificed Zinc: Zinc bars need replacement before complete corrosion for prolonged protection
Galvanising
- Galvanising: A method where iron is shielded by a zinc coating to prevent rusting
- Process: It involves electroplating or zinc immersion
- ZnCO3 Formation: Zinc reacts with oxygen and CO2, creating a protective barrier
- Sacrificial Protection: Even if the coating is damaged, iron is safeguarded from rusting
Question for GalvanisationTry yourself: In sacrificial protection, why is a more reactive metal attached to a less reactive metal?View Solution
The document Galvanisation | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Galvanisation - Chemistry for GCSE/IGCSE - Class 10
| 1. What is galvanising and how does it provide sacrificial protection? |  |
Ans. Galvanising is the process of coating iron or steel with a layer of zinc to protect it from corrosion. Zinc is more reactive than iron, so it corrodes before the iron does, acting as a sacrificial anode and protecting the underlying metal.
| 2. How long does galvanised protection last? |  |
Ans. The lifespan of galvanised protection depends on the thickness of the zinc coating, the environment it is exposed to, and the quality of the application. In general, galvanised coatings can last anywhere from 50 to 100 years in most environments.
| 3. What are some common applications of galvanised sacrificial protection? |  |
Ans. Galvanised sacrificial protection is commonly used in industries such as construction, automotive, agriculture, and marine to protect metal structures, equipment, vehicles, and components from corrosion.
| 4. Can galvanised protection be applied to any metal surface? |  |
Ans. Galvanising can be applied to iron and steel surfaces, as zinc has a strong affinity for these metals. However, it may not be suitable for non-ferrous metals like aluminum, copper, or stainless steel.
| 5. Is galvanising a cost-effective method of protection against corrosion? |  |
Ans. Yes, galvanising is considered a cost-effective method of protecting metal surfaces from corrosion. While the initial cost may be higher than other methods, the long lifespan and low maintenance requirements make it a cost-effective choice in the long run.
Related Searches















