Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Extraction of Metals
Extraction of Metals | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Extraction of Metals
- The Earth's crust is rich in metals and metal compounds like gold, copper, iron oxide, and aluminium oxide.
- Metals of value are commonly found in chemical compounds, known as ores.
- An ore is a solid material containing a sufficient amount of metal to make its extraction economically viable.
- The extraction of metals from their ores involves various methods such as electrolysis, using a blast furnace, or reacting with more reactive substances.
- In the case of metal oxide ores, the extraction process is a reduction reaction where oxygen is removed.
- Examples of oxide ores include hematite (iron ore) and bauxite (aluminium ore).
- Some metals, like gold and platinum, exist in nature as uncombined elements due to their low reactivity.
- These unreactive metals, known as native metals, can be directly mined from the Earth's crust.
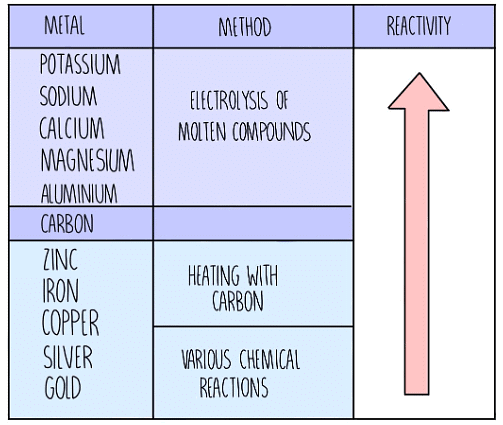
- The extraction method for a metal depends on its position in the reactivity series.
- Metals higher in the reactivity series require processes like electrolysis for extraction, while those lower down can be extracted by heating with carbon.
The Extraction Method Depends on the Position of a Metal in the Reactivity Series
Question for Extraction of MetalsTry yourself: What is the extraction method for metals that are higher in the reactivity series?View Solution
The document Extraction of Metals | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Extraction of Metals - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the process of extraction of metals? |  |
Ans. The extraction of metals involves various processes such as mining, crushing, concentration, smelting, and refining to obtain the pure metal from its ore.
| 2. What are some common methods used in the extraction of metals? |  |
Ans. Common methods used in the extraction of metals include hydrometallurgy, pyrometallurgy, and electrometallurgy, depending on the type of ore and metal being extracted.
| 3. How does smelting play a role in the extraction of metals? |  |
Ans. Smelting is a process where the ore is heated with a reducing agent to remove the metal from the ore. This process is crucial in extracting metals like iron, copper, and lead.
| 4. What is the importance of refining in the extraction of metals? |  |
Ans. Refining is essential in the extraction of metals as it helps remove impurities and other elements to obtain a pure form of the metal. This ensures the metal meets the required standards for various applications.
| 5. Can you explain the difference between ferrous and non-ferrous metals in the context of extraction? |  |
Ans. Ferrous metals contain iron, while non-ferrous metals do not. The extraction processes for these metals differ due to their chemical properties, with ferrous metals typically requiring more extensive refining processes.
Related Searches















