Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Formulas and Functional Groups
Formulas and Functional Groups | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Displayed Formulae
- Organic Chemistry involves examining the arrangement, properties, and reactions of carbon-based compounds.
- Carbon-containing compounds are categorized as organic compounds.
- Substances like metal carbonates, carbon dioxide, and carbon monoxide are not classified as organic compounds.
- Many structures you draw in organic chemistry are hydrocarbons, compounds made of hydrogen and carbon.
- Hydrocarbons exclusively consist of hydrogen and carbon atoms.
- Organic compounds can be depicted in various forms, such as Displayed Formulae, General Formulae, and Structural Formulae.
- The displayed formula illustrates the spatial arrangement of atoms and bonds within a molecule.
- For example:
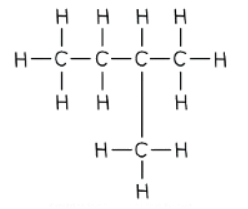
- The displayed formula provides vital information about the compound:
- It has 5 carbon atoms
- It has 12 hydrogen atoms
- It consists of only single bonds
Question for Formulas and Functional GroupsTry yourself: Which type of formula provides information about the spatial arrangement of atoms and bonds within a molecule?View Solution
Structural Formulae
- In structural formulae, the emphasis is on providing enough information for clarity while omitting most covalent bonds.
- Important bonds like double and triple bonds are always depicted.
- Identical groups are often grouped together within brackets for simplification.
- Side groups are represented using brackets.
- Straight chain alkanes are typically illustrated in a specific format.
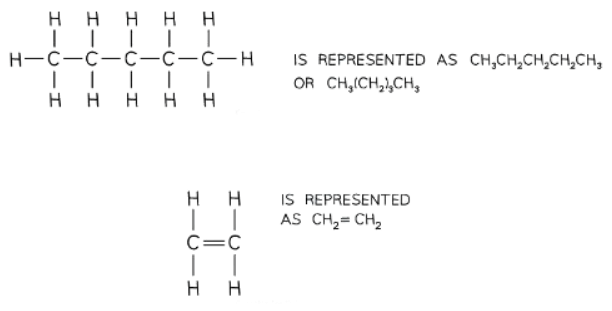
Structural Isomers
- Structural isomers possess the same molecular formula but differ in their structural arrangements.
- The molecular formula indicates the precise number of atoms for each element in a compound.
- Compounds sharing the same molecular formula can exhibit distinct structural formulae due to varying spatial atom arrangements.
- Below are two instances highlighting structural isomerism:
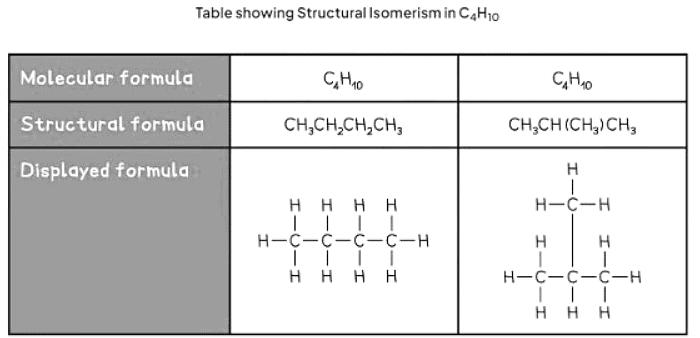
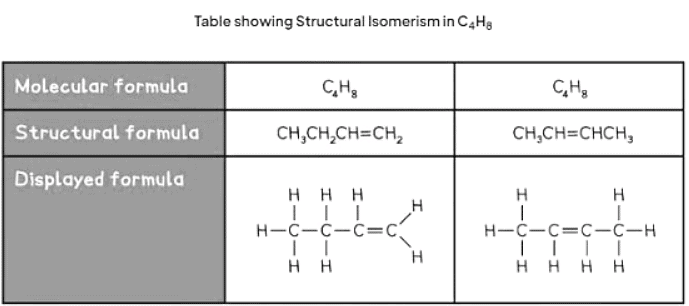
The document Formulas and Functional Groups | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Formulas and Functional Groups - Chemistry for GCSE/IGCSE - Class 10
| 1. What are displayed formulae in organic chemistry? |  |
Ans. Displayed formulae in organic chemistry show all the atoms and bonds in a molecule, with each atom shown individually along with its bonding arrangement.
| 2. How are structural formulae different from general formulae in organic chemistry? |  |
Ans. Structural formulae in organic chemistry show the arrangement of atoms and bonds in a molecule, while general formulae represent a class of compounds by a specific formula without showing the arrangement of atoms.
| 3. What is structural isomerism in organic compounds? |  |
Ans. Structural isomerism in organic compounds refers to compounds that have the same molecular formula but different structural arrangements of atoms, leading to different chemical properties.
| 4. How many structural isomers are possible for C4H10? |  |
Ans. There are two structural isomers possible for C4H10, which are butane and isobutane.
| 5. Can you explain the concept of functional groups in organic compounds? |  |
Ans. Functional groups are specific groups of atoms within a molecule that determine the chemical properties and reactions of the compound. They are responsible for the characteristic reactions and properties of organic compounds.
Related Searches





















