Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Saturated and Unsaturated Compounds
Saturated and Unsaturated Compounds | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Saturated & Unsaturated Compounds
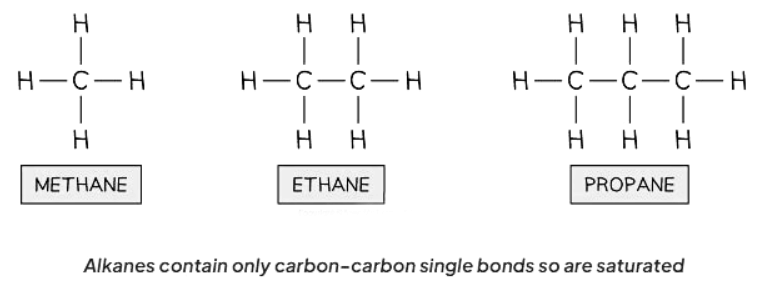
- Saturated compounds consist of molecules where all carbon-carbon bonds are single bonds.
- Alkanes are examples of saturated compounds.
- Alkanes are hydrocarbons with a saturated structure and follow the general formula CnH2n+2.
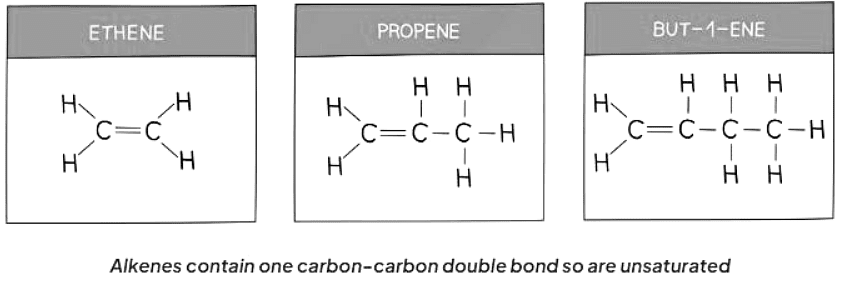
- Unsaturated compounds are characterized by molecules where one or more carbon-carbon bonds are not single bonds.
- Alkenes are examples of unsaturated compounds.
- Alkenes are hydrocarbons with an unsaturated structure, following the general formula CnH2n.
- The presence of a double bond, C=C, in alkenes enables them to form additional bonds with other atoms by breaking the C=C bond and allowing incoming atoms to create another single bond with each carbon atom of the functional group.
- Each carbon atom in the functional group forms four single bonds instead of one double bond and two single bonds.
Question for Saturated and Unsaturated CompoundsTry yourself: Which of the following compounds is an example of a saturated compound?View Solution
The document Saturated and Unsaturated Compounds | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Saturated and Unsaturated Compounds - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the difference between saturated and unsaturated compounds? |  |
Ans. Saturated compounds contain only single bonds between carbon atoms, while unsaturated compounds contain one or more double or triple bonds between carbon atoms.
| 2. How can we determine if a compound is saturated or unsaturated? |  |
Ans. One way to determine if a compound is saturated or unsaturated is by examining its chemical structure. If there are only single bonds between carbon atoms, it is saturated. If there are double or triple bonds present, it is unsaturated.
| 3. Can saturated compounds form double or triple bonds? |  |
Ans. No, saturated compounds cannot form double or triple bonds because they already have the maximum number of single bonds between carbon atoms.
| 4. What are some examples of saturated compounds? |  |
Ans. Examples of saturated compounds include alkanes such as methane, ethane, and propane.
| 5. What are some examples of unsaturated compounds? |  |
Ans. Examples of unsaturated compounds include alkenes such as ethene, propene, and butene, as well as alkynes such as ethyne and propyne.
Related Searches
















