Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Fuels & Separation of Petroleum
Fuels & Separation of Petroleum | Chemistry for GCSE/IGCSE - Class 10 PDF Download
| Table of contents |

|
| Common Fossil Fuels |

|
| Petroleum & Fractional Distillation |

|
| Fractional Distillation |

|
| Key Concepts in Hydrocarbon Properties |

|
Common Fossil Fuels
- A fuel is a substance that, upon combustion, releases heat energy.
- This released heat energy can be converted into electricity, which is extensively used in our daily lives.
- The most prevalent fossil fuels comprise coal, natural gas, and hydrocarbons like methane and propane, derived from crude oil.
- Hydrocarbons exclusively consist of hydrogen and carbon atoms.
- Methane, represented as CH4, stands as the primary component of natural gas.
Petroleum & Fractional Distillation
Petroleum
- Petroleum, also known as crude oil, is a complex mixture of hydrocarbons along with natural gas.
- It is a thick, sticky, black liquid found under porous rock formations, whether underground or under the sea.
- Diagram showing crude oil under the sea:
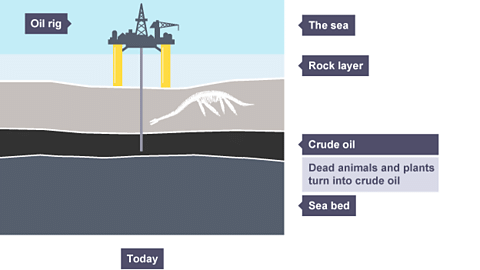
- Petroleum, as a mixture, is not directly useful, but its components, known as fractions, have various applications. These fractions are separated through a process called fractional distillation.
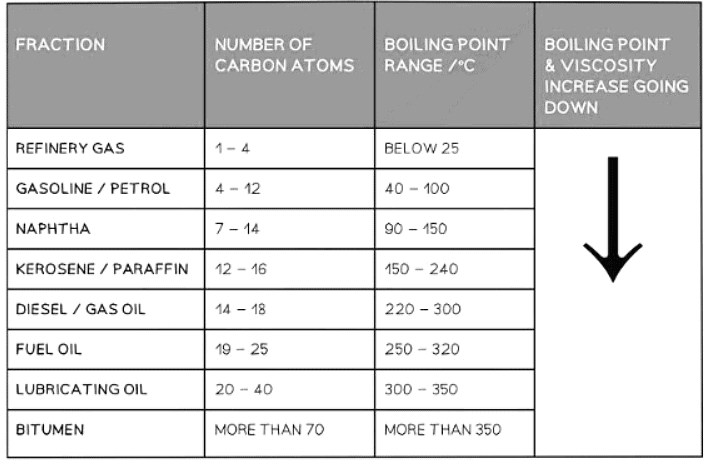
- During fractional distillation, fractions in petroleum are separated based on their similar properties and boiling points, which are influenced by the number of carbon atoms in their molecular chains.
- The boiling point and viscosity of each fraction increase as the length of the carbon chain grows.
Question for Fuels & Separation of PetroleumTry yourself: What is the primary component of natural gas?View Solution
Fractional Distillation
Diagram showing the process of fractional distillation: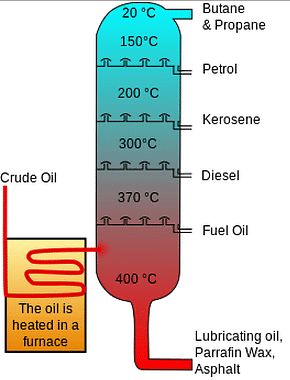
- Fractional distillation takes place in a fractionating column.
- The fractionating column is hot at the bottom and cools at the top.
- When crude oil is introduced into the column, it is heated, causing vapors to rise.
- Vapors of hydrocarbons with high boiling points condense into liquids and are collected at the bottom.
- Vapors of hydrocarbons with low boiling points rise to the top of the column and are collected there.
- Various fractions condense at different heights based on their boiling points and are collected as liquids.
- Fractions with smaller hydrocarbons are gathered at the top as gases.
- Fractions with larger hydrocarbons are collected in the lower sections of the column.
Properties of Fractions
Fractions obtained through fractional distillation exhibit distinct properties:
- Volatility: Refers to how easily a substance vaporizes. For instance, lighter fractions have higher volatility compared to heavier ones.
- Boiling Points: Different fractions have varying boiling points, leading to their separation during distillation. For instance, gasoline has a lower boiling point than diesel.
- Viscosity: This property determines the fluidity of a fraction. Lighter fractions are less viscous than heavier ones. For example, kerosene is more viscous than gasoline.
- Flammability: Some fractions are more flammable than others due to their chemical composition. For example, lighter fractions like gasoline are highly flammable compared to heavier fractions like lubricating oil.
Key Concepts in Hydrocarbon Properties
Viscosity
- Viscosity pertains to how easily a liquid flows.
- Liquids with high viscosity are thick and flow less readily.
- When the number of carbon atoms in a hydrocarbon chain increases, the attraction between the molecules intensifies, leading to a higher viscosity as the chain lengthens.
- Consequently, liquids become more viscous with increasing molecular mass, causing them to flow less easily.
Colour
- The color of a liquid darkens as the carbon chain lengthens, signifying increased thickness and viscosity.
Melting Point/Boiling Point
- Larger molecules exhibit greater intermolecular attraction, requiring more heat to separate them.
- Consequently, as molecular size increases, so does the boiling point.
Volatility
- Volatility denotes a substance's tendency to vaporize.
- As molecular size grows, hydrocarbon liquids become less volatile due to heightened intermolecular attraction with increasing molecular size.
Uses of Various Fractions
- Refinery gas: used for heating and cooking purposes
- Gasoline: serves as fuel for automobiles (petrol)
- Naphtha: utilized as a raw material for chemical production
- Kerosene: essential for jet fuel production (paraffin)
- Diesel: functions as fuel for diesel engines (gas oil)
- Fuel oil: used for powering ships and home heating
- Lubricating oil: necessary for lubricants, polishes, and waxes
- Bitumen: employed in road surfacing applications
Trends in Chemical Properties
Question for Fuels & Separation of PetroleumTry yourself: Which property determines the fluidity of a fraction obtained through fractional distillation?View Solution
The document Fuels & Separation of Petroleum | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Fuels & Separation of Petroleum - Chemistry for GCSE/IGCSE - Class 10
| 1. What is fractional distillation and how is it used in the separation of petroleum? |  |
Ans. Fractional distillation is a process used to separate different components of a mixture based on their boiling points. In the case of petroleum, fractional distillation is used to separate the various hydrocarbons present in crude oil by heating the oil to boiling point and then collecting the different fractions as they vaporize at different temperatures.
| 2. What are some common fossil fuels besides petroleum? |  |
Ans. Some common fossil fuels besides petroleum include coal and natural gas. These fossil fuels are also formed from the remains of plants and animals that were buried and subjected to high pressure and heat over millions of years.
| 3. What are some key properties of hydrocarbons that make them useful as fuels? |  |
Ans. Hydrocarbons are organic compounds made up of hydrogen and carbon atoms. They have high energy content, burn easily, and produce large amounts of heat when combusted, making them ideal for use as fuels in various applications.
| 4. How does the fractional distillation process work to separate petroleum into different components? |  |
Ans. In fractional distillation, crude oil is heated in a fractionating column, causing it to vaporize. The vapor rises through the column, cooling as it goes higher. Different hydrocarbons condense at different heights in the column based on their boiling points, allowing for the separation of the components.
| 5. Why is the separation of petroleum into different fractions important in the production of various products? |  |
Ans. The separation of petroleum into different fractions is important because each fraction has unique properties and uses. By separating petroleum into its components, different fractions can be used to produce a wide range of products such as gasoline, diesel, jet fuel, and lubricants.
Related Searches














