Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Addition and Condensation Polymers
Addition and Condensation Polymers | Chemistry for GCSE/IGCSE - Class 10 PDF Download
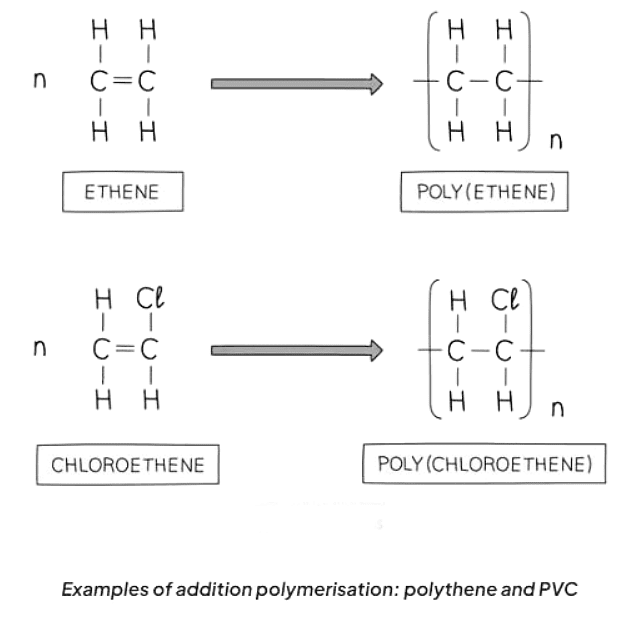
Addition Polymers
- Addition polymers result from the bonding together of numerous monomers and are exclusive to monomers featuring C=C bonds.
- Within each C=C bond, one of the bonds is broken, forming a connection with the adjacent monomer, leading to the formation of a polymer characterized by single bonds exclusively.
- Many polymers can be synthesized through the addition of alkene monomers, while others originate from alkene monomers featuring diverse atoms attached, like chlorine or a hydroxyl group.
- To name the polymer, the monomer's name is enclosed in brackets, with "poly-" prefixed to it. For instance, using propene as the alkene monomer results in the polymer being named poly(propene).
- Poly(ethene) is created through the addition polymerization of ethene monomers.
Deducing the Polymer from the Monomer
- Polymer molecules are significantly larger compared to most other molecules.
- Repeat units are utilized when representing the formula of a polymer.
- To depict a repeat unit, convert the double bond in the monomer to a single bond in the repeat unit.
- Include a bond at each end of the repeat unit, with the bonds extending outside the brackets (known as extension or continuation bonds).
- Use a small subscript 'n' at the bottom right to indicate a large number of repeat units.
- Add the remaining groups in the same sequence as they encircled the double bond in the monomer.
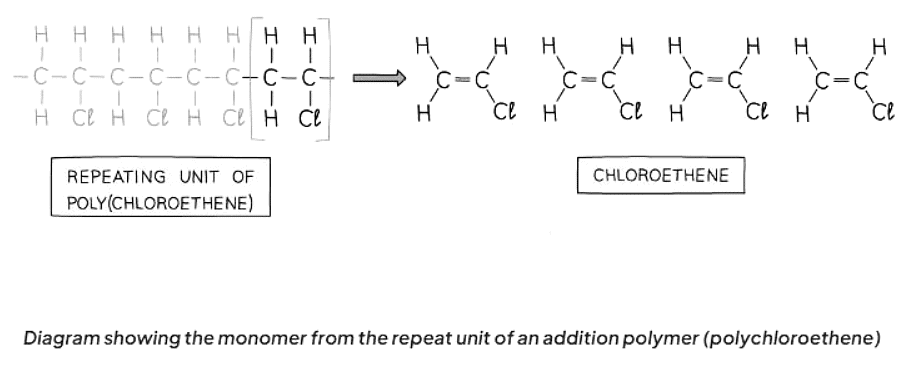
Deducing the Monomer from the Polymer
- Identify the repeating unit in the polymer.
- Convert the single bond in the repeat unit to a double bond in the monomer.
- Eliminate the bond from each end of the repeat unit.
Question for Addition and Condensation PolymersTry yourself: Which type of bonds are exclusive to monomers in the formation of addition polymers?View Solution
Condensation Polymers
- Condensation polymers are created by linking two distinct monomers together with the elimination of a small molecule, typically water.
- This process contrasts with addition polymers, where polymer molecules form exclusively.
- During condensation polymerization, one water molecule is produced for each polymer linkage.
- Monomers involved in condensation polymers possess two functional groups each, located at opposite ends.
- These functional groups react with one another, forming extended chains of alternating monomers that constitute the polymer.
- Reversing the reaction by hydrolysis (adding water) under acidic conditions breaks the peptide link, yielding the original monomers.
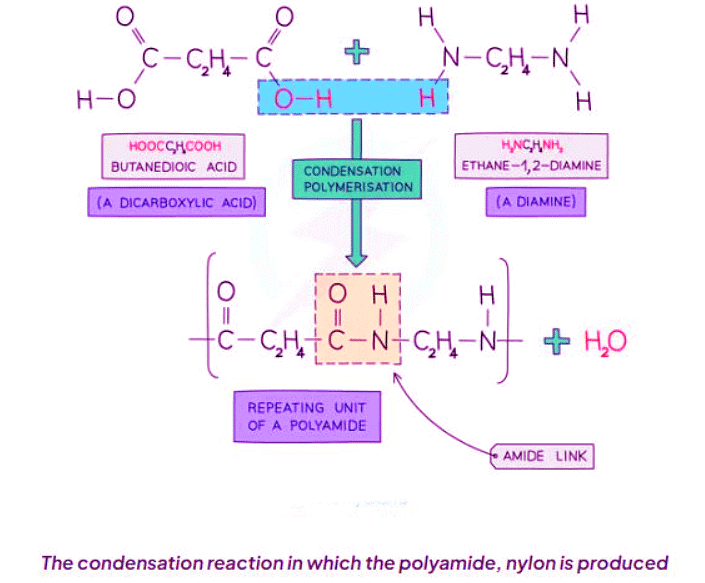
Forming Nylon
- Nylon, a polyamide, is synthesized from dicarboxylic acid monomers, which contain a carboxylic group (-COOH) at both ends, and diamines, which feature an amino group (-NH2) at each end.
- The -COOH groups of one monomer react with the -NH2 groups of another monomer.
- This reaction results in the formation of an amide linkage, accompanied by the removal of a water molecule for each bond.
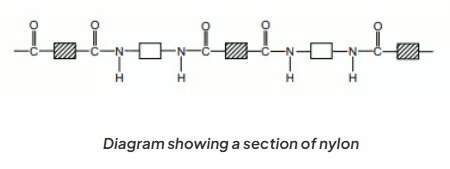
- The arrangement of nylon can be illustrated by depicting the polymer with rectangular shapes to symbolize the carbon chains.
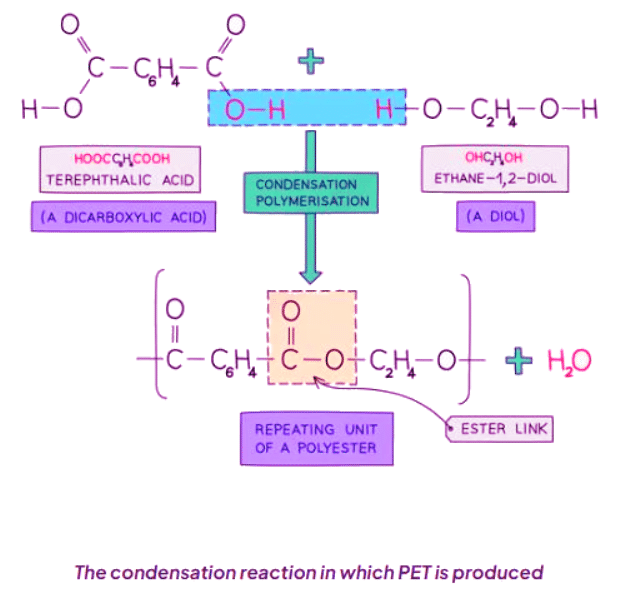
Forming Polyesters
- PET, or polyethylene terephthalate, is a type of polyester.
- It is derived from dicarboxylic acid monomers and diols.
- Each -COOH group in the monomers reacts with an -OH group from another monomer.
- This reaction forms an ester linkage and releases a water molecule.
- Condensation polymerization, responsible for PET formation, produces one water molecule for each ester linkage.
- PET is commonly used in synthetic fibers and is marketed under the brand name terylene.
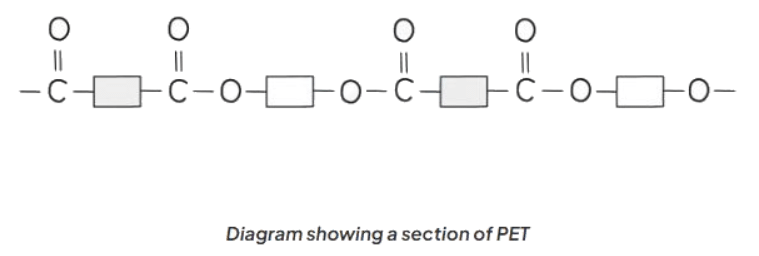
- The structure of PET, along with other polyesters, can be depicted by illustrating the polymer using boxes to symbolize the carbon chains.
- This method can be applied universally to represent the structures of various polyesters.
Question for Addition and Condensation PolymersTry yourself: How are condensation polymers formed?View Solution
The document Addition and Condensation Polymers | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Addition and Condensation Polymers - Chemistry for GCSE/IGCSE - Class 10
| 1. What are addition polymers? |  |
Ans. Addition polymers are formed through the repeated addition of monomers without the elimination of any byproducts. Examples include polyethylene and polypropylene.
| 2. What are condensation polymers? |  |
Ans. Condensation polymers are formed through a reaction between two different monomers, resulting in the elimination of a small molecule like water or alcohol. Examples include polyamide and polyester.
| 3. How is Polyethylene Terephthalate (PET) produced? |  |
Ans. PET is produced by the condensation polymerization of terephthalic acid and ethylene glycol, resulting in a strong, lightweight, and recyclable polymer commonly used in the production of plastic bottles.
| 4. What are the main differences between addition and condensation polymers? |  |
Ans. Addition polymers are formed by the repeated addition of monomers, while condensation polymers are formed by a reaction between two different monomers with the elimination of a small molecule. Additionally, addition polymers do not require a catalyst, while condensation polymers often do.
| 5. What are the key properties of addition and condensation polymers? |  |
Ans. Addition polymers typically have high molecular weights, are chemically inert, and have good thermal and electrical insulating properties. On the other hand, condensation polymers often have better mechanical properties, are biodegradable, and can be more easily modified through chemical reactions.
Related Searches















