Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Chromatography
Chromatography | Chemistry for GCSE/IGCSE - Class 10 PDF Download
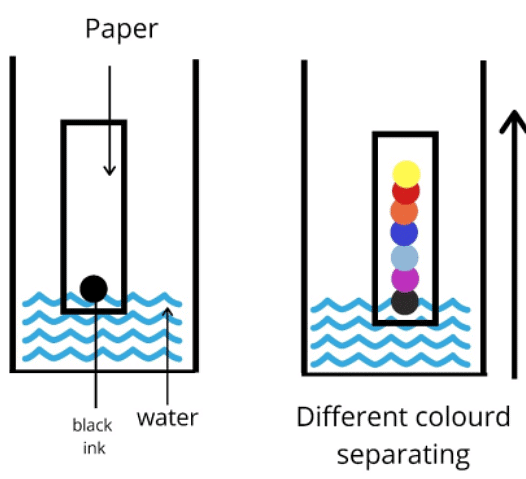
Paper Chromatography
- This technique, known as paper chromatography, is employed for separating substances based on their differing solubilities in a specific solvent. For instance, it can be used to separate different colored inks mixed to create black ink.
- To initiate the process, a line is drawn using a pencil on chromatography paper, and small spots of the sample are carefully placed on this line. Pencil is preferred for this step to prevent the ink from blending with the samples during the chromatography process.
- Subsequently, the paper is immersed into a solvent container, ensuring that the pencil line remains above the solvent level to prevent the samples from dissolving into the solvent.
- Through capillary action, the solvent ascends the paper, carrying some of the colored components along with it.
- As different substances exhibit varying solubilities, they travel at different rates, leading to their separation on the paper. Substances with higher solubility move a greater distance than those with lower solubility.
- This method effectively reveals the distinct constituents present in the ink or dye mixture.
- Analysis of the composition of ink using paper chromatography:
Interpret Simple Chromatograms
- When multiple substances are identical, they will generate chromatograms that are indistinguishable.
- Incorporating a recognized compound as a reference point is customary, aiding in the identification of unknown spots.
- Mixtures will segregate on the paper, revealing individual components as distinct spots.
- Impurities will manifest with multiple spots, whereas pure substances should exhibit only one spot.
Question for ChromatographyTry yourself: What is the purpose of drawing a line using a pencil on the chromatography paper before conducting paper chromatography?View Solution
The document Chromatography | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Chromatography - Chemistry for GCSE/IGCSE - Class 10
| 1. What is paper chromatography? |  |
Ans. Paper chromatography is a technique used to separate and analyze mixtures of substances based on their different solubilities and attraction to the paper and solvent.
| 2. How does paper chromatography work? |  |
Ans. In paper chromatography, a sample is placed on a piece of paper and allowed to travel up the paper as a solvent moves through it. The different components of the sample will move at different rates, resulting in separation.
| 3. What is the purpose of using paper chromatography? |  |
Ans. Paper chromatography is commonly used in chemistry labs to identify and analyze the components of a mixture, such as inks, dyes, and amino acids. It is also used in forensic science and pharmaceutical industries.
| 4. What factors can affect the results of paper chromatography? |  |
Ans. Factors such as the type of paper used, the solvent mixture, the temperature, and the amount of sample applied can all impact the results of a paper chromatography experiment.
| 5. Can paper chromatography be used to separate colored compounds? |  |
Ans. Yes, paper chromatography is particularly useful for separating colored compounds, as the different colors will be visible as they travel up the paper strip and can be easily identified and analyzed.
Related Searches















