Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Identification of Anions
Identification of Anions | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Identification of Anions
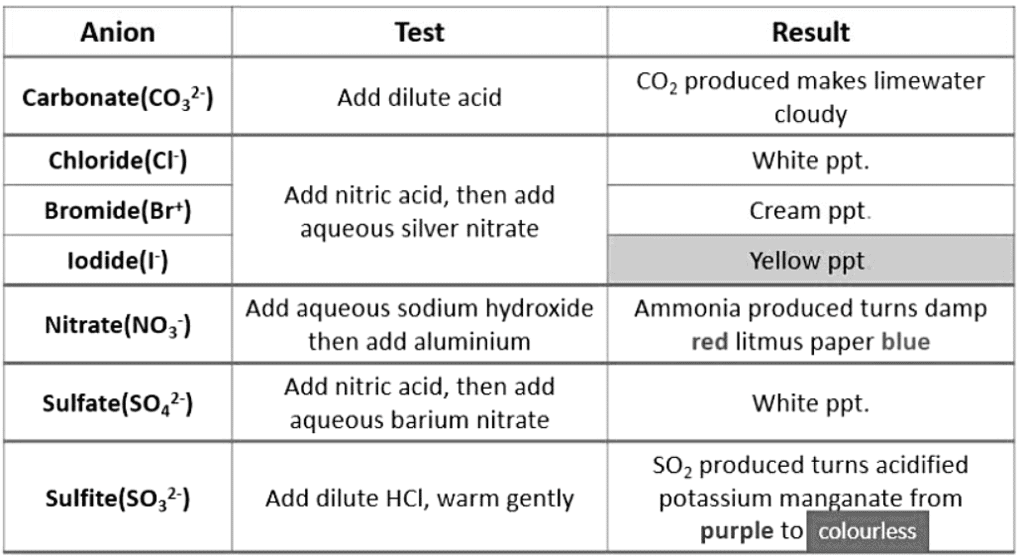
Acidification with aqueous silver nitrate and aqueous barium nitrate/chloride is performed to eliminate ions that could lead to incorrect positive outcomes.
- The most frequently removed ion is carbonate ion.
- The choice of acid needs to be carefully considered to prevent it from affecting the results.
- For instance, avoid acidifying aqueous silver nitrate solution with hydrochloric acid as it will produce a white precipitate due to the chloride ion present in the acid.
- Similarly, avoid acidifying aqueous barium nitrate/chloride solution with sulfuric acid as it will result in a white precipitate due to the sulfate ion present in the acid.
Question for Identification of AnionsTry yourself: What is the most frequently removed ion during the process of acidification using silver nitrate and barium nitrate/chloride?View Solution
The document Identification of Anions | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Identification of Anions - Chemistry for GCSE/IGCSE - Class 10
| 1. What are anions? |  |
Ans. Anions are negatively charged ions that are formed when an atom gains one or more electrons.
| 2. How are anions identified in a laboratory setting? |  |
Ans. Anions can be identified through various chemical tests such as precipitation reactions, flame tests, and complexation reactions.
| 3. What are some common anions that are tested for in analytical chemistry? |  |
Ans. Common anions that are tested for in analytical chemistry include chloride (Cl-), sulfate (SO4 2-), carbonate (CO3 2-), nitrate (NO3 -), and phosphate (PO4 3-).
| 4. Why is it important to accurately identify anions in a sample? |  |
Ans. Accurate identification of anions is important in various fields such as environmental monitoring, water treatment, and pharmaceutical analysis as they can provide valuable information about the composition and purity of a sample.
| 5. Can anions be identified using instrumental techniques? |  |
Ans. Yes, anions can also be identified using instrumental techniques such as ion chromatography, mass spectrometry, and infrared spectroscopy for more precise and reliable results.
Related Searches















