Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Identification of Cations
Identification of Cations | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Identification of Cations
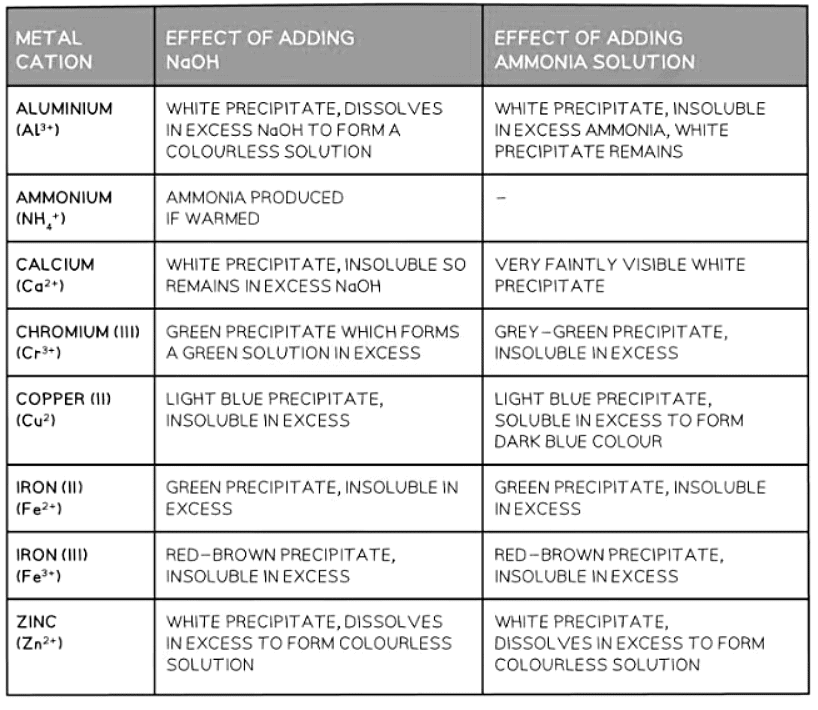
- Metal cations dissolved in water can be distinguished by the hue of the solid they produce when sodium hydroxide or ammonia is introduced.
- Typically, with a small quantity of NaOH, the metal hydroxide solidifies and separates from the solution.
- In cases of excessive NaOH, some of the solids formed may dissolve again.
- Initially, a few drops of NaOH are introduced, and any color alterations or solid formations are recorded.
- Subsequently, excess NaOH is added, and the reaction is observed once more.
- This procedure is repeated for the test using ammonia solution.
Analysing results
- The results for each cation in the syllabus are detailed below.
- If a precipitate forms with NaOH or aqueous ammonia, it indicates the hydroxide's insolubility in water.
- Example with Zinc chloride:
- ZnCl2(aq) + 2NaOH(aq) → Zn(OH)2(s) + 2NaCl(aq)
- Calcium ions (Ca2+) can be differentiated from Zinc (Zn2+) and Aluminum (Al3+) ions because calcium hydroxide precipitate remains insoluble in excess NaOH, unlike zinc and aluminum hydroxide.
- Zinc ions (Zn2+) can be distinguished from Aluminum ions (Al3+) as Zn(OH)2 dissolves in excess aqueous ammonia while Al(OH)3 does not.
- Most transition metals form hydroxides with distinct colors.
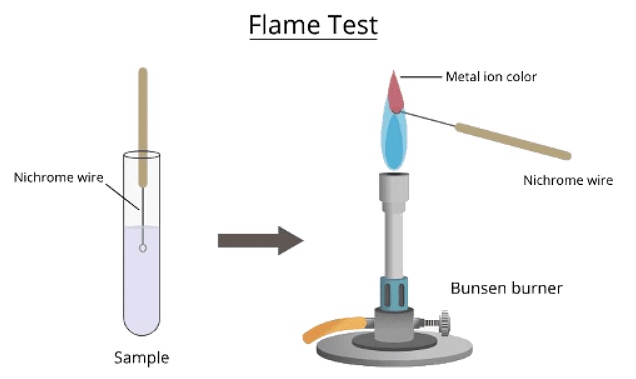
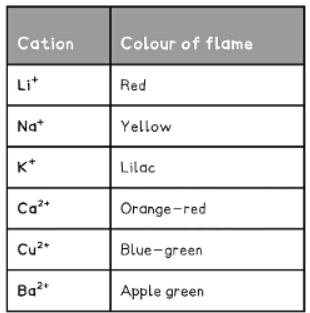
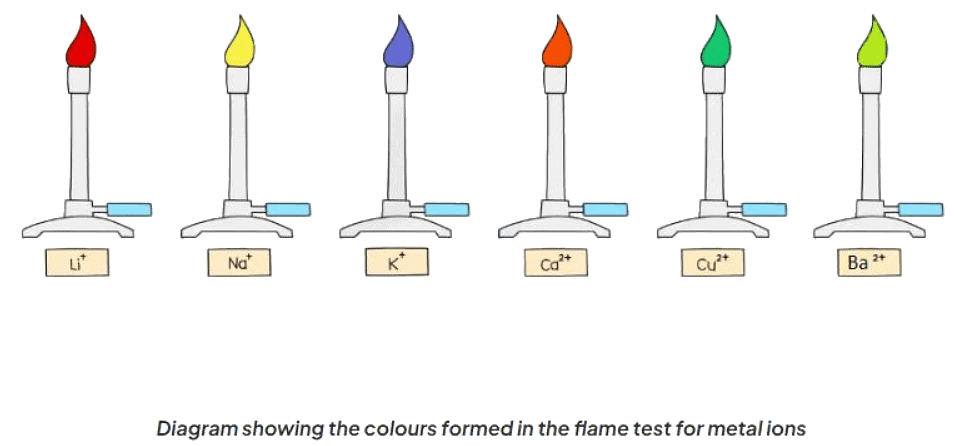
- The flame test is employed to recognize metal cations based on the hue of the flame they generate.
- Different metal ions produce distinct colors.
- Immerse a loop made of a non-reactive metal wire like nichrome or platinum in concentrated acid, then expose it to the blue flame of a Bunsen burner until no color change occurs.
- This step is crucial to ensure the presence of only one type of ion, as multiple ions can blend colors, leading to inaccurate identification.
- Cleansing the wire loop in this manner helps prevent contamination.
- Apply a small amount of the compound onto a non-reactive metal wire loop like nichrome or platinum.
- Submerge the loop into the solid sample or solution, then position it at the edge of the blue Bunsen flame.
- Be cautious not to overheat the wire to the point where it emits a red glow, which could cause confusion with flame coloration.
- Diagram showing the technique for carrying out a flame test:
- The colour of the flame is observed and used to identify the metal ion present:
Question for Identification of CationsTry yourself: What is the purpose of adding a small quantity of NaOH in the identification of metal cations?View Solution
The document Identification of Cations | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Identification of Cations - Chemistry for GCSE/IGCSE - Class 10
| 1. What are cations in the context of chemical analysis? |  |
Ans. Cations are positively charged ions that are formed when an atom loses one or more electrons during a chemical reaction. In the context of chemical analysis, cations are identified and separated based on their unique properties and behavior in various tests.
| 2. How are cations identified in a chemical analysis? |  |
Ans. Cations are identified in a chemical analysis through a series of qualitative tests that exploit their specific reactions with reagents. These tests include flame tests, precipitation reactions, and complexation reactions that help distinguish between different cations based on their characteristic properties.
| 3. Why is it important to identify cations in chemical analysis? |  |
Ans. Identifying cations in chemical analysis is crucial for determining the composition of a sample and understanding its properties. It helps in qualitative analysis, identifying unknown substances, and ensuring the purity of a compound. Additionally, cation identification is essential in various fields such as environmental monitoring, forensic science, and pharmaceutical research.
| 4. What are some common cations that are tested for in chemical analysis? |  |
Ans. Common cations that are tested for in chemical analysis include sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), iron (Fe2+ and Fe3+), copper (Cu2+), and lead (Pb2+). These cations are often found in a variety of compounds and play crucial roles in biological and industrial processes.
| 5. What techniques are used to separate and identify cations in chemical analysis? |  |
Ans. Techniques such as flame tests, precipitation reactions, complexation reactions, and spectroscopic methods (such as atomic absorption spectroscopy and X-ray fluorescence) are commonly used to separate and identify cations in chemical analysis. These methods exploit the unique properties of cations to differentiate between different ions present in a sample.
Related Searches




















