Best Study Material for Year 11 Exam
Year 11 Exam > Year 11 Notes > Physics for GCSE/IGCSE > Gases & Absolute Temperature
Gases & Absolute Temperature | Physics for GCSE/IGCSE - Year 11 PDF Download
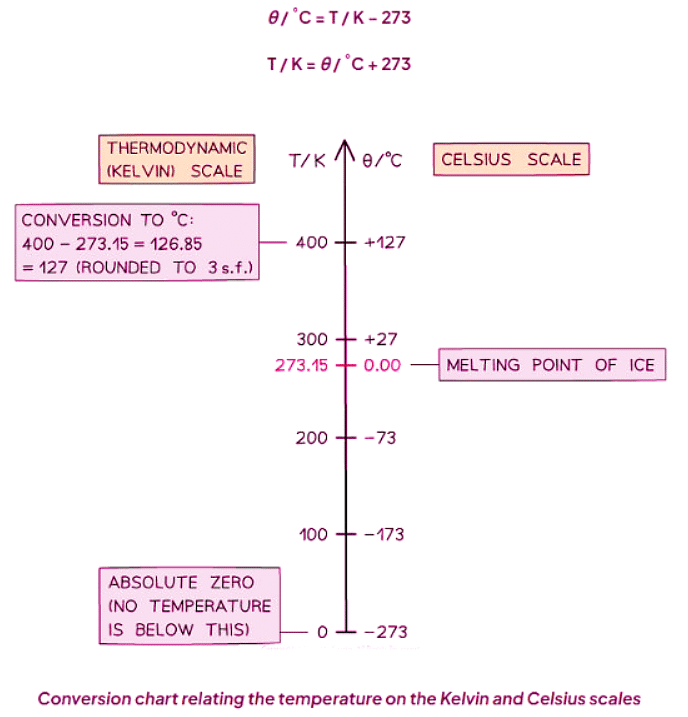
Absolute Temperature
- The Kelvin temperature scale commences at absolute zero.
- At this point, 0 K corresponds to -273 °C.
- A rise of 1 K equates to the same alteration as a rise of 1 °C.
- Temperatures below 0 K are unattainable, signifying that Kelvin temperatures cannot be negative.
- To convert between Celsius (θ) and Kelvin (T) scales, utilize the subsequent conversion:
The Gas Laws
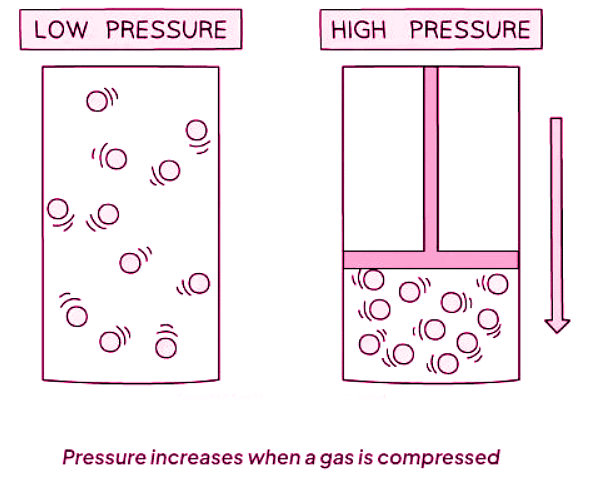
If the temperature of a gas remains constant, changes in pressure occur when the gas is compressed or expanded:
- Compressed: Decreasing the volume increases the pressure.
- Expanded: Increasing the volume decreases the pressure.
- Likewise, alterations in pressure can induce variations in volume.
- A vacuum pump has the capability to extract air from a sealed enclosure.
- The illustration below illustrates how the volume of a inflated balloon alters when the surrounding air pressure diminishes:
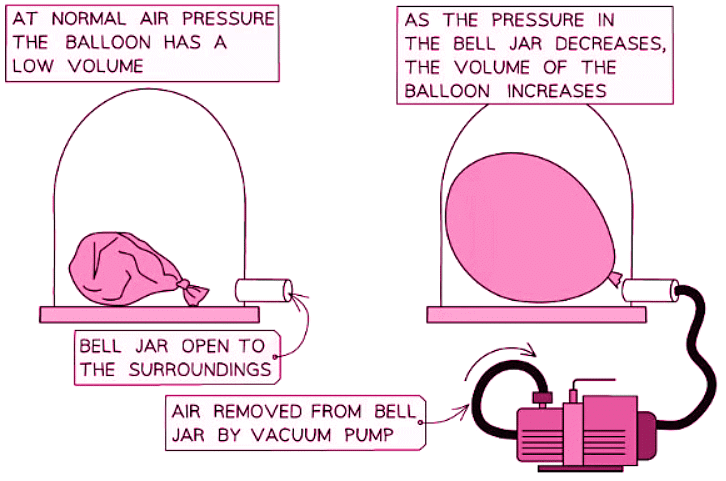
- When gas is compressed, its molecules will collide with the container walls more often.
- This results in a greater total force exerted on the walls, consequently raising the pressure.
 |
Test: Kinetic Particle Model of Matter - 1
|
Start Test |
Start Test
Pressure & Temperature (Constant Volume)
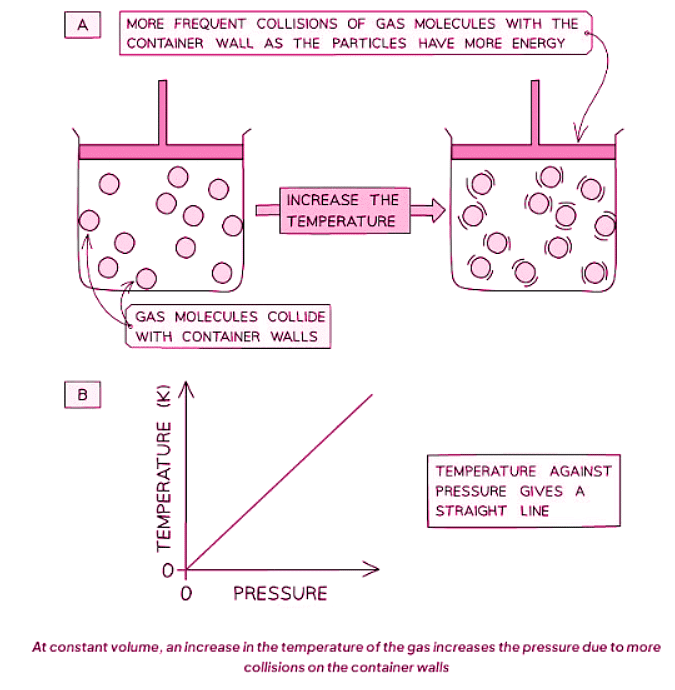
- The velocity of molecules rises with temperature escalation, and diminishes when temperature decreases.
- Given that average kinetic energy is contingent on velocity, an increase in temperature, while keeping volume constant, also augments the kinetic energy of molecules.
- Consequently, hotter gases exhibit higher average kinetic energy, whereas cooler ones manifest lower average kinetic energy.
- As gas heats up, its molecules accelerate, leading to more frequent collisions with container walls and consequent pressure escalation.
- Hence, at a constant volume, heightened temperature augments gas pressure, and conversely.
- Diagram A illustrates how molecules in a fixed volume collide more frequently with container walls as temperature rises.
- Diagram B portrays a linear graph since temperature and pressure are directly proportional.
Question for Gases & Absolute Temperature
Try yourself:
What is the relationship between temperature and pressure in a gas at constant volume?View Solution
Boyle's Law
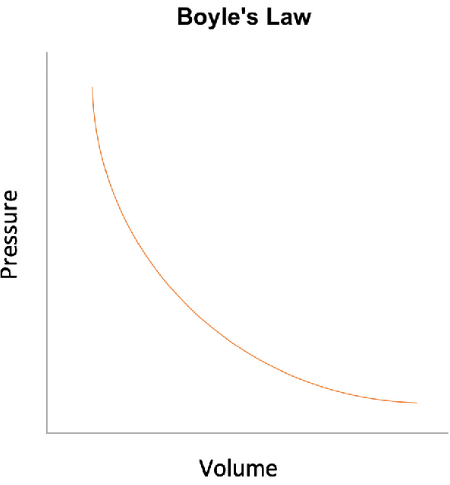
- Boyle's Law describes the relationship between the pressure and volume of an ideal gas when the temperature remains constant.
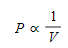 This means that as the pressure on a gas increases, its volume decreases, and vice versa.
This means that as the pressure on a gas increases, its volume decreases, and vice versa. - The relationship between pressure and volume for a fixed mass of gas at a constant temperature can be expressed as:
P1V1 = P2V2 - Here,
- P1 = initial pressure (Pa)
- P2 = final pressure (Pa)
- V1 = initial volume (m3)
- V2 = final volume (m3)
- It's important to note that the units of volume are typically in cubic meters (m3) and pressure in Pascals (Pa).
- Calculations involving different units, such as cm3 or MPa, can be directly converted as long as the final answer is given in the original units.
The document Gases & Absolute Temperature | Physics for GCSE/IGCSE - Year 11 is a part of the Year 11 Course Physics for GCSE/IGCSE.
All you need of Year 11 at this link: Year 11
|
127 videos|148 docs|35 tests
|
FAQs on Gases & Absolute Temperature - Physics for GCSE/IGCSE - Year 11
| 1. What is absolute temperature and how is it different from Celsius temperature? |  |
| 2. How do you convert Kelvin to Celsius temperature? |  |
Ans. To convert Kelvin to Celsius, you subtract 273 from the Kelvin temperature. For example, if the temperature is 300K, the Celsius equivalent would be 27°C (300K - 273 = 27°C).
| 3. How are gas volume and pressure related according to the gas laws? |  |
Ans. According to Boyle's Law, the volume of a gas is inversely proportional to its pressure when the temperature is constant. This means that as pressure increases, volume decreases, and vice versa.
| 4. Can you explain the relationship between temperature and pressure in gases? |  |
Ans. According to Charles's Law, the volume of a gas is directly proportional to its temperature when the pressure is constant. This means that as temperature increases, volume increases, and vice versa.
| 5. How does understanding gas behavior help in real-life applications? |  |
Ans. Understanding gas behavior is crucial in various industries such as manufacturing, healthcare, and transportation. It helps in gas storage, pressure regulation, and ensuring safety measures are in place.
Related Searches