Year 11 Exam > Year 11 Notes > Physics for GCSE/IGCSE > Melting & Boiling
Melting & Boiling | Physics for GCSE/IGCSE - Year 11 PDF Download
Fixed Points of Water
- The melting and boiling temperatures of pure water are termed as fixed points.
- Ice melts at 0 °C.
- Pure water reaches its boiling point at 100 °C.
- These values are acknowledged as the standard temperatures for pure water under atmospheric pressure.

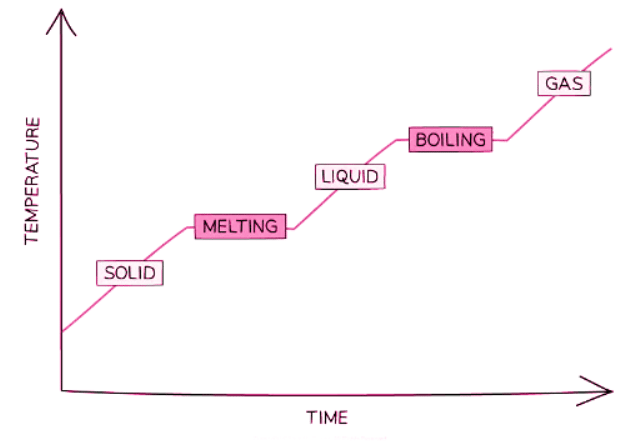
Melting & Boiling
- When a substance changes state, it can either melt or freeze, boil or condense.
- During this change, the substance does not change temperature, even as energy is transferred to or away from its thermal energy store.
- For instance, when liquid water is heated, its temperature rises until it reaches the boiling point. Even with added thermal energy, the water does not get hotter, indicating that the internal energy is not increasing.
Boiling
- Heating liquid water with thermal energy, such as from a gas flame or kettle element, causes its temperature to increase until it reaches the boiling point.
- At the boiling point, further addition of thermal energy doesn't raise the temperature of the liquid water.
- Instead, the extra thermal energy is utilized to overcome the intermolecular forces between water molecules.
- As these forces are surpassed, the liquid water transforms into water vapor, known as evaporation or vaporization.
- Conversely, during cooling, energy transfer occurs in reverse.
- Gas transforms back into liquid through condensation.
Melting
- When solid water (ice) is heated by thermal energy, either from the surroundings or a flame, it undergoes melting.
- At the melting point, additional thermal energy doesn't elevate the temperature of the solid water.
- This indicates that the internal energy remains constant.
- The extra thermal energy is utilized to overcome the intermolecular forces within the solid ice molecules.
- As these forces are overcome, the solid water transitions into a liquid state, known as melting.
- Conversely, during cooling, heat transfer occurs in reverse.
- Liquid transforms back into solid through freezing.
Question for Melting & BoilingTry yourself: What happens to the temperature of liquid water when it reaches its boiling point?View Solution
Condensation & Solidification
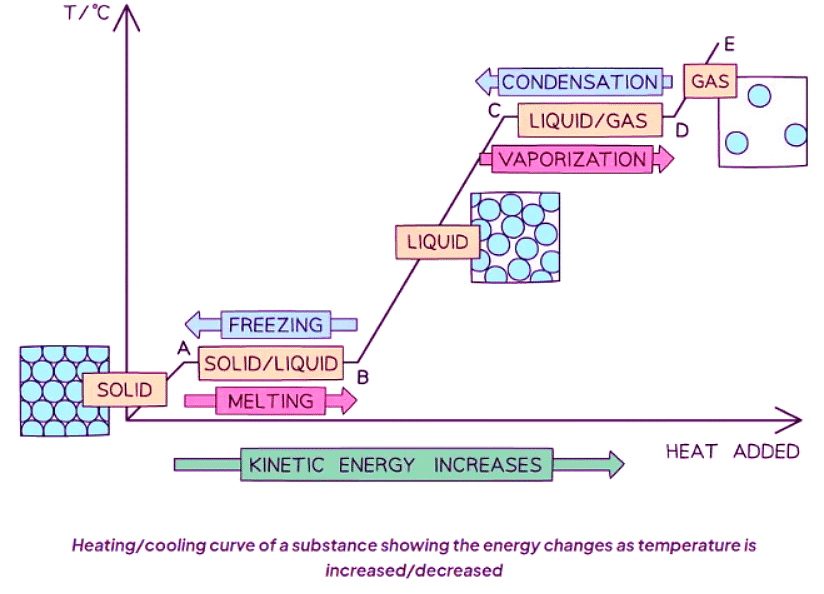
- Heating and cooling graphs serve to depict:
- The temperature variations of a substance as energy is transferred to or from it.
- Locations of state changes within the substance.
- Heating and cooling graphs typically exhibit similar patterns.
- Heating involves energy transfer into the system, leading to increased kinetic energy of molecules (indicated by red arrows pointing right).
- Cooling involves energy transfer out of the system or dissipation to the surroundings, resulting in decreased kinetic energy of molecules (indicated by blue arrows pointing left).
Condensation
- Particle diagrams alongside the graph demonstrate the process of a gas condensing into a liquid.
- Before condensation, the gas has already released heat energy, causing it to cool down.
- The particles within the gas lose kinetic energy, resulting in slower movement.
- With reduced energy, they cannot overcome the intermolecular forces between molecules.
- Consequently, the particles draw closer to each other.
- They retain adequate energy only to flow over one another.
- The gas transforms into a liquid without any temperature alteration.
Solidification
- Particle diagrams accompanying the graph illustrate the process of a liquid solidifying into a solid.
- Before solidification, the liquid has already released heat energy, resulting in cooling.
- The particles within the liquid decrease in kinetic energy, leading to slower motion.
- Their energy becomes insufficient to overcome the intermolecular forces between molecules.
- Consequently, the particles move closer together.
- They retain adequate energy solely to vibrate about their fixed positions.
- The liquid transforms into a solid without any alteration in temperature.
The document Melting & Boiling | Physics for GCSE/IGCSE - Year 11 is a part of the Year 11 Course Physics for GCSE/IGCSE.
All you need of Year 11 at this link: Year 11
|
127 videos|148 docs|35 tests
|
FAQs on Melting & Boiling - Physics for GCSE/IGCSE - Year 11
| 1. What are the fixed points of water? |  |
Ans. The fixed points of water are the temperatures at which water undergoes phase changes. The melting point of water is 0 degrees Celsius, while the boiling point is 100 degrees Celsius at standard atmospheric pressure.
| 2. What is the process of evaporation and condensation? |  |
Ans. Evaporation is the process by which a liquid turns into a gas at a temperature below its boiling point. Condensation is the process by which a gas turns into a liquid at a temperature below its boiling point.
| 3. How does melting occur? |  |
Ans. Melting is the process by which a solid turns into a liquid when sufficient heat is applied. The temperature at which a solid melts is known as the melting point.
| 4. What is the difference between condensation and solidification? |  |
Ans. Condensation is the process by which a gas turns into a liquid, while solidification is the process by which a liquid turns into a solid. Both processes involve a change in state due to a change in temperature.
| 5. How does heating and cooling affect changes in state? |  |
Ans. Heating causes substances to change from a solid to a liquid and then to a gas, while cooling causes the reverse process. Changes in state occur due to the absorption or release of heat energy.
Related Searches





















