Class 10 Exam > Class 10 Notes > Physics for GCSE/IGCSE > The Atom
The Atom | Physics for GCSE/IGCSE - Class 10 PDF Download
Atomic Structure
- Atoms constitute the fundamental units of all substances.
- They possess minute dimensions, with a radius of merely 1 × 10-10 m.
- Approximately one hundred million atoms could align along your thumbnail's width.
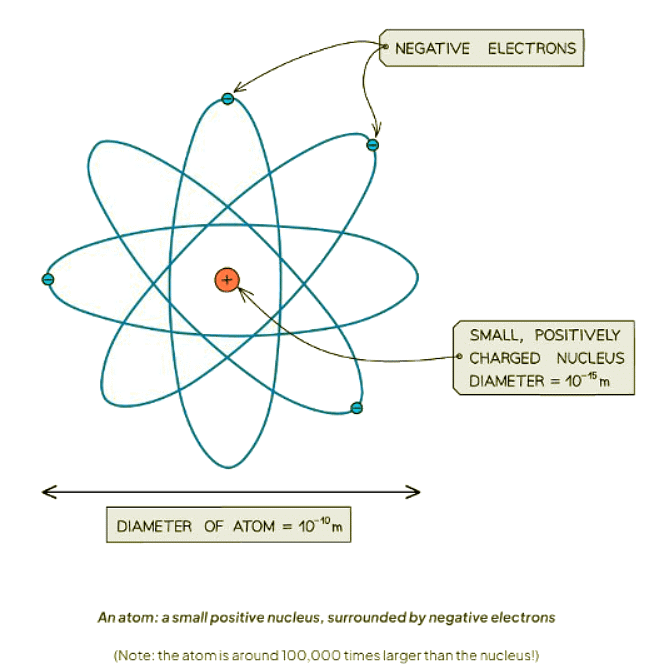
- Atoms feature a minuscule yet dense nucleus positioned at their core, orbited by electrons.
- The nucleus, which is over 10,000 times smaller than the atom itself, contains nearly all of the atom's mass.
- Atoms consist of compact, positively charged nuclei, encompassed by negatively charged electrons.
Rutherford's Experiment
Overview of the Experiment
- In 1909, a team of scientists led by physicist Ernest Rutherford conducted an experiment to investigate the Plum Pudding model of the atom. Rutherford directed two of his students, Hans Geiger and Ernest Marsden, to carry out the experiment.
- The experiment involved the scattering of alpha (α) particles by a thin metal sheet, supporting the nuclear model of the atom.
- A beam of alpha particles (He 2 ions) was aimed at a thin gold foil, with the expectation that the alpha particles would pass through the foil, possibly changing direction slightly.
- Surprisingly, the results revealed that:
- Most of the alpha particles passed straight through the foil.
- Some alpha particles changed direction but still passed through the foil.
- A few alpha particles rebounded off the gold foil.
- The unexpected behavior of the alpha particles, especially the rebounding, contradicted the Plum Pudding model, necessitating the development of a new model. This marked a significant milestone in understanding the structure of the atom.
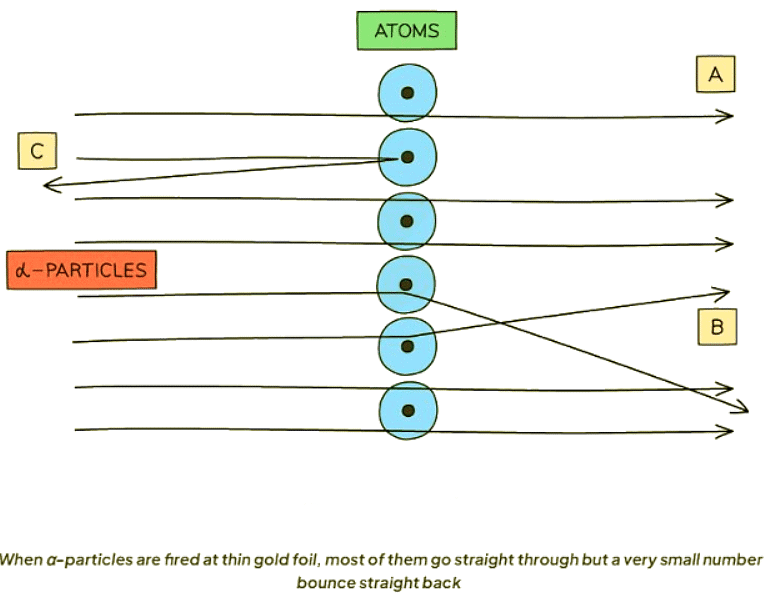
- When α-particles are directed at thin gold foil:
- Most α-particles pass through without deviation (A).
This occurrence is attributed to the predominant empty space within the atom. - Some α-particles undergo deflection at slight angles (B).
This phenomenon arises due to the repulsion between the positively charged α-particles and the positively charged nucleus, which houses the majority of the atom's mass. - A minute fraction of α-particles recoil directly backward (C).
This rare event occurs owing to the nucleus's exceedingly minute size.
- Most α-particles pass through without deviation (A).
Question for The AtomTry yourself: What did Rutherford's experiment on alpha particle scattering reveal about the Plum Pudding model of the atom?View Solution
Atoms & Ions
- Atoms and ions are tiny particles that are the building blocks of matter.
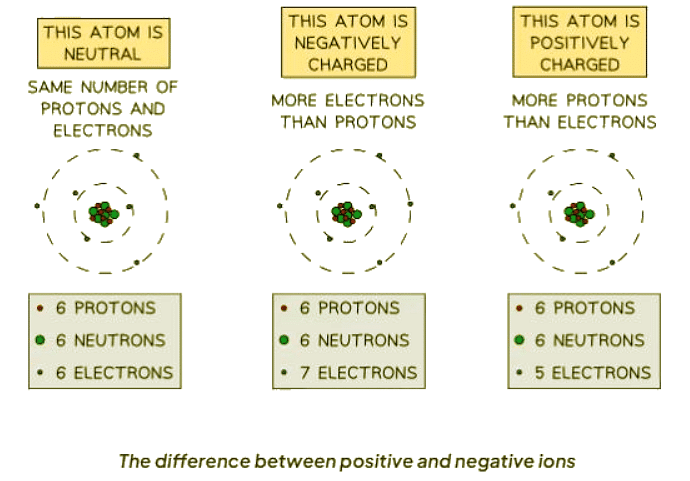
- Ions are atoms or groups of atoms that have an electric charge due to the loss or gain of electrons.
- When atoms lose electrons, they become positively charged ions, as there are more protons than electrons.
- Conversely, when atoms gain electrons, they become negatively charged ions, with more electrons than protons.
- Atoms undergo these changes to achieve stability.
- A stable atom has an equal number of protons (positive charge) and electrons (negative charge), making it electrically neutral.
- For example, sodium (Na) can lose an electron to become a positively charged sodium ion (Na+).
- Similarly, chlorine (Cl) can gain an electron to become a negatively charged chloride ion (Cl-).
The document The Atom | Physics for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Physics for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
126 videos|182 docs|35 tests
|
FAQs on The Atom - Physics for GCSE/IGCSE - Class 10
| 1. What is the atomic model proposed by Ernest Rutherford? |  |
Ans. Ernest Rutherford proposed the atomic model known as the Rutherford model, which suggested that atoms have a dense, positively charged nucleus surrounded by negatively charged electrons.
| 2. What is the summary of Ernest Rutherford's Gold Foil Experiment? |  |
Ans. In Ernest Rutherford's Gold Foil Experiment, alpha particles were directed at a thin gold foil. Most of the alpha particles passed through the foil, but some were deflected at large angles, leading to the discovery of the nucleus.
| 3. How did Rutherford's experiment contribute to our understanding of the structure of the atom? |  |
Ans. Rutherford's experiment showed that atoms have a small, dense nucleus at their center, which contains positively charged protons, while negatively charged electrons orbit the nucleus.
| 4. What is the difference between atoms and ions? |  |
Ans. Atoms are neutral particles with an equal number of protons and electrons. Ions, on the other hand, are charged particles that have gained or lost electrons, resulting in a net positive or negative charge.
| 5. How does the structure of the atom relate to the concept of positive and negative ions? |  |
Ans. Positive ions are formed when atoms lose electrons, resulting in a net positive charge, while negative ions are formed when atoms gain electrons, leading to a net negative charge. This relates to the structure of the atom as the number of protons and electrons determines the overall charge of the ion.
Related Searches
















