Class 10 Exam > Class 10 Notes > Physics for GCSE/IGCSE > Types of Radiation
Types of Radiation | Physics for GCSE/IGCSE - Class 10 PDF Download
Radioactive Decay
- Some atomic nuclei are unstable
- Unstable nuclei result from imbalances in forces within the nucleus. These forces exist between particles in the nucleus and are often due to an excess of protons or neutrons.
- The presence of excess protons or neutrons within the nucleus leads to the imbalance of forces between particles.
- Carbon-14, an isotope of carbon, exemplifies instability with two extra neutrons compared to stable carbon-12.
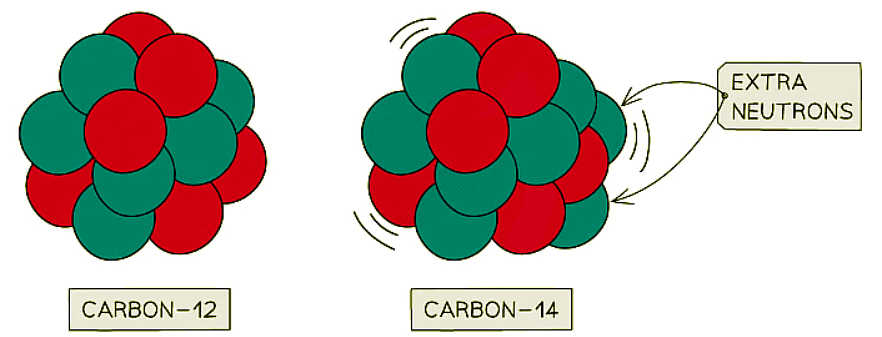 Carbon-12 is stable, whereas carbon-14 is unstable. This is because carbon-14 has two extra neutrons
Carbon-12 is stable, whereas carbon-14 is unstable. This is because carbon-14 has two extra neutrons - Certain isotopes become unstable due to their significant size or an imbalance in the number of neutrons.
- To achieve stability, unstable nuclei emit radiation.
- This radiation can manifest as either high-energy particles or waves.
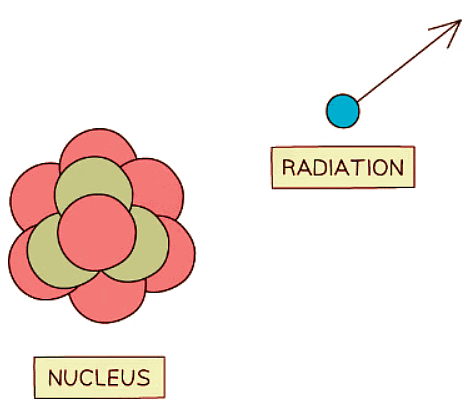 Unstable nuclei decay by emitting high energy particles or waves
Unstable nuclei decay by emitting high energy particles or waves
- As radiation disperses from the nucleus, it carries away some energy, diminishing the nucleus's overall energy and rendering it more stable.
- This process, known as radioactive decay, is random in nature, making it impossible to precisely predict when a specific nucleus will decay.
- The unpredictability arises because any nucleus has an equal likelihood of decaying, and the timing of decay cannot be determined.
- Furthermore, the rate of decay remains constant regardless of external conditions, making it only possible to estimate the probability of nuclei decaying within a given timeframe.
- Consequently, the emission of radiation is spontaneous, occurring randomly in direction.
Types of Radioactive Decay
- Radioactive decay occurs when an unstable nucleus emits nuclear radiation.
- There are three main types of radiation that can be emitted:
- Alpha (α) particles
- Beta (β-) particles
- Gamma (γ) radiation
- These changes are spontaneous and random
Alpha Particles
- Alpha particles, represented by the symbol α, are identical to helium nuclei.
- Comprising two neutrons and two protons, they possess a charge of +2 and thus can be influenced by an electric field.
Beta Particles
- Beta particles, symbolized by β, are fast-moving electrons.
- They are generated in nuclei when a neutron decays into a proton and an electron.
- Beta particles carry a charge of -1, making them responsive to electric fields.
Gamma Rays
- Gamma rays, represented by γ, are electromagnetic waves.
- They possess the highest energy among various electromagnetic waves.
- Gamma rays are uncharged.
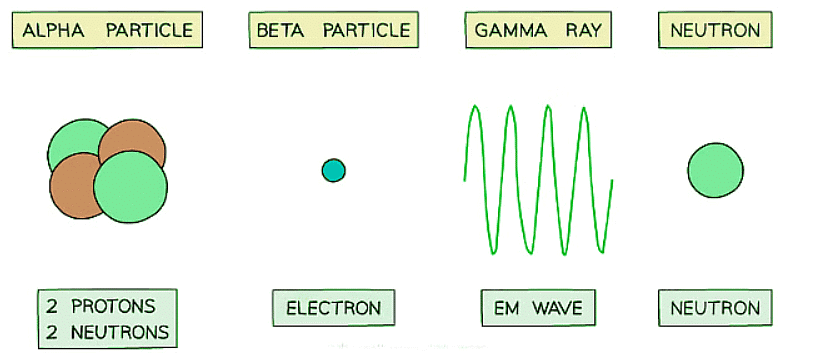 Alpha particles, beta particles and gamma waves can be emitted from unstable nuclei
Alpha particles, beta particles and gamma waves can be emitted from unstable nuclei
Question for Types of RadiationTry yourself: Which type of radiation is represented by electromagnetic waves?View Solution
Alpha, Beta & Gamma Emission
- Nature: Alpha, beta, and gamma radiation can be distinguished by understanding the type of particle or radiation they are.
- Relative Ionising Effects: This refers to how easily these radiation types can ionize other atoms.
- Relative Penetrating Abilities: It indicates how far alpha, beta, and gamma radiation can travel before being completely stopped.
- The properties of Alpha, Beta and Gamma: These properties are presented in a table and further elaborated below.
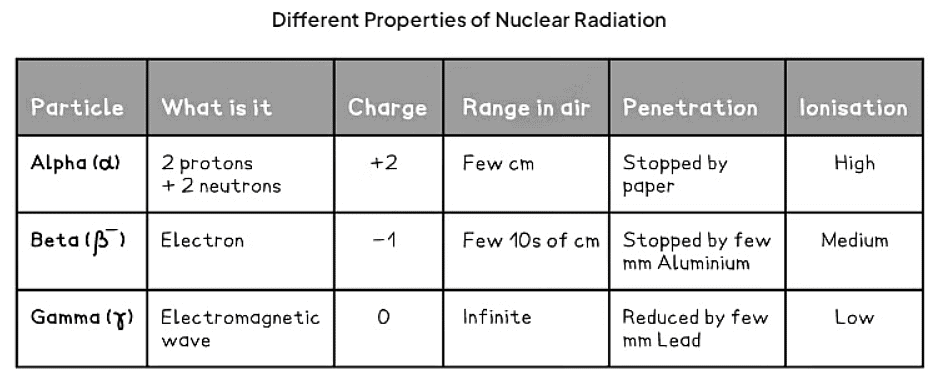
- The trend down the table indicates:
- The range increases.
- Penetrating power increases.
- Ionisation decreases.
Penetrating Power
- Alpha, beta, and gamma radiation possess distinct properties.
- They penetrate materials differently, affecting the materials they interact with.
- Alpha radiation has the least penetrating power, while gamma radiation is the most penetrating.
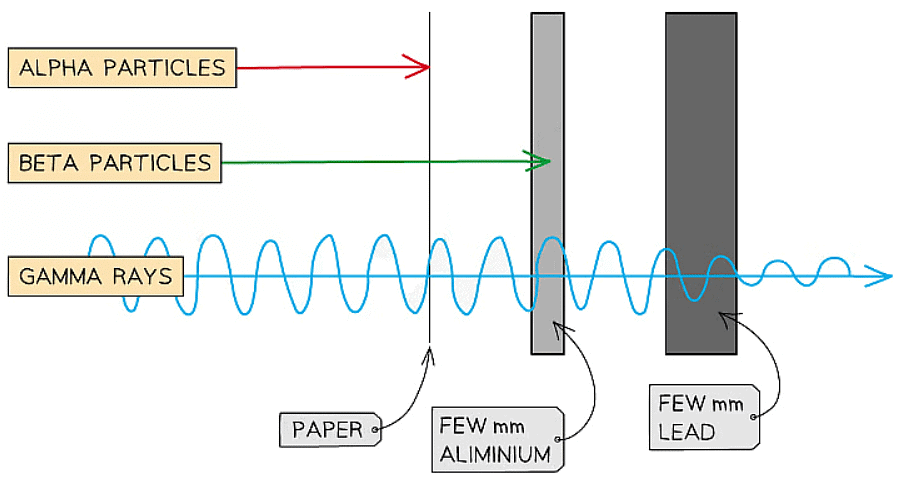 Alpha, beta and gamma are different in how they penetrate materials. Alpha is the least penetrating, and gamma is the most penetrating
Alpha, beta and gamma are different in how they penetrate materials. Alpha is the least penetrating, and gamma is the most penetrating
- Alpha radiation is effectively stopped by paper, whereas beta and gamma rays can pass through it.
- Beta radiation requires only a few millimeters of aluminum to be stopped, while gamma rays can penetrate aluminum.
- Gamma rays are only partially obstructed by thick lead.
The document Types of Radiation | Physics for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Physics for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
126 videos|182 docs|35 tests
|
FAQs on Types of Radiation - Physics for GCSE/IGCSE - Class 10
| 1. What is radioactive decay and how does it relate to nucleus stability? |  |
Ans. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation. Nuclei are considered unstable when they have an imbalance of protons and neutrons, leading to excess energy that needs to be released through decay in order to achieve a more stable configuration.
| 2. What are the different types of radioactive decay and how do they differ from each other? |  |
Ans. The main types of radioactive decay are alpha, beta, and gamma decay. In alpha decay, an alpha particle (helium nucleus) is emitted from the nucleus. Beta decay involves the emission of either a beta-minus particle (an electron) or a beta-plus particle (a positron). Gamma decay releases gamma rays, high-energy electromagnetic radiation.
| 3. How does the emission of alpha, beta, and gamma particles affect the properties of radiation? |  |
Ans. Alpha particles have the least penetration power but the highest ionization power. Beta particles have higher penetration power than alpha particles but lower ionization power. Gamma rays have the highest penetration power and are the most damaging to living tissues due to their ability to penetrate deeply.
| 4. What are the trends observed in the emission of alpha, beta, and gamma radiation down the periodic table? |  |
Ans. As you move down the periodic table, the emission of alpha particles becomes less common as the stability of the nuclei increases. Beta decay is more common in heavier elements due to the presence of excess neutrons. Gamma radiation is more commonly emitted by nuclei with high energy levels.
| 5. How does atomic stability play a role in determining the likelihood of radioactive decay in a nucleus? |  |
Ans. Atoms with unstable nuclei are more likely to undergo radioactive decay in order to achieve a more stable configuration. Factors such as the ratio of protons to neutrons, the presence of excess energy, and the overall balance of the nucleus contribute to the likelihood of decay occurring.
Related Searches















