Year 7 Exam > Year 7 Notes > Density of Solids, Liquids and Gases
Density of Solids, Liquids and Gases - Year 7 PDF Download
| Table of contents |

|
| Introduction |

|
| Calculating density |

|
| Measuring density |

|
| Finding the volume of a regular shape |

|
| Finding the volume of an irregular shape |

|
| Densities of the states |

|
| Expanding and Contracting |

|
Introduction
- The density of an object or substance is determined by its mass divided by its volume. Density reflects how closely packed the particles within a substance are. When particles are tightly packed, the density is higher compared to when they are more spread out.
- Density is a measure of how closely particles are arranged within a substance. It is calculated by dividing the mass of the substance by its volume. Mass refers to the amount of matter in an object and is typically measured in kilograms (kg), while volume represents the space occupied by an object, usually measured in cubic meters (m3) or cubic centimeters (cm3).
- The units for density vary based on the units used for mass and volume. Commonly used units include grams per cubic centimeter (g/cm3) and kilograms per cubic meter (kg/m3).
- The density of a substance is influenced by the mass of its particles and how closely they are packed together. As substances change state or experience temperature variations, their density can also change accordingly.
Calculating density
- The weight of an object can vary even when objects are the same size due to differences in density. For instance, an iron cube is heavier than a wooden cube and a polystyrene cube of the same size because iron has a higher density.
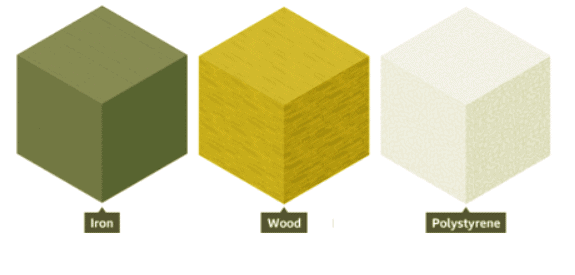
- Objects with higher density feel heavier because there is more mass packed into the same space.
- Density is commonly measured in grams per cubic centimeter (g/cm3) or kilograms per cubic meter (kg/m3).
- The density of an object is its mass divided by its volume, calculated using the formula:
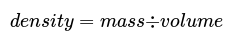
Measuring density
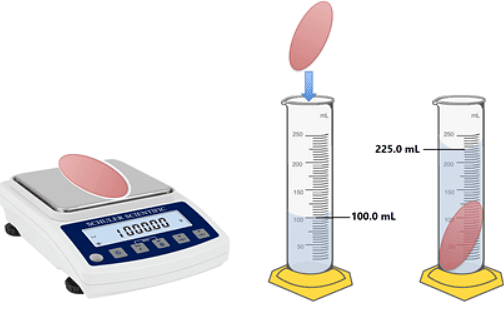
- The two quantities you need to measure in order to work out the density of an object are:
- Mass: A measure of the amount of matter an object is made out of. Mass is measured in kilograms (kg).
- Volume: The amount of space an object occupies, usually measured in m3 or cm3.
- To measure mass, place the object on a top pan balance and read off the mass.
Finding the volume of a regular shape
- Finding the volume of a regular shape can be determined through direct measurement techniques. For instance, in the case of a cuboid, the volume can be calculated by multiplying the length by the width by the height.
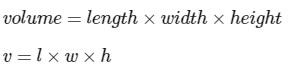
Finding the volume of an irregular shape
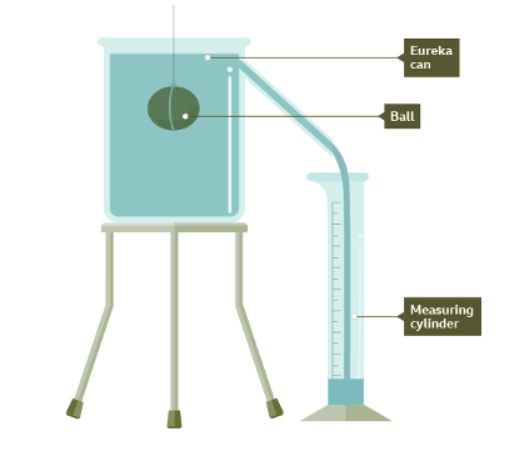
- To calculate the volume of an irregular object that cannot be measured conventionally, a eureka can be employed.
- A eureka can is filled with water, and by submerging the object in it, the displaced water is collected and measured to determine the object's volume.
- The principle behind this method is that the volume of water displaced is equal to the volume of the object.
Question for Density of Solids, Liquids and GasesTry yourself: What is the formula for calculating density?View Solution
Densities of the states
Different materials possess varying densities.
Here are some examples:
- Solid steel: 7.82 g/cm3
- Liquid water: 1.00 g/cm3
- Air (gaseous form): 0.0012 g/cm3
The density of a substance is influenced by the mass of its particles and how closely packed they are. For instance, solid steel has the highest density, air has the lowest, and liquid water falls in between.
The density of a substance depends both on the mass of the particles , and how closely spaced they are.
- Water, ice and water vapour are the same substance in different states. Each one has a different density.
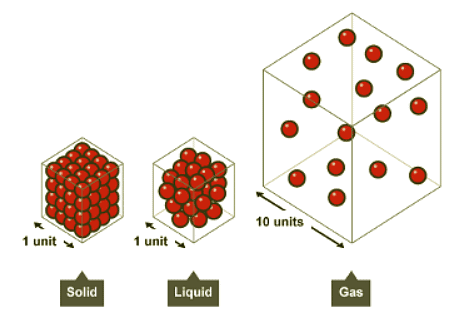
- In solids, the particles are tightly packed, which accounts for their high density. On the other hand, gases have low densities because their particles are spread out and have more space between them.
Solids
- The particles in solids are closely packed together, leading to high density.
Liquids
- In liquids, particles are also close together but arranged randomly, resulting in a slightly lower density than solids.
- For instance:
- The density of solid aluminium is 2.72 g/cm³.
- The density of liquid aluminium is 2.38 g/cm³.
- This difference in density explains why liquid aluminium floats on top of solid aluminium.
- Water behaves uniquely as its solid form (ice) is less dense than its liquid form due to particle expansion upon freezing.
- Consequently, icebergs and ice cubes float in liquid water.
Gases
- The particles in gases are widely spaced apart, resulting in very low density.
Expanding and Contracting
Expanding
- When an object or substance is heated, its particles vibrate more vigorously, causing them to push each other apart, leading to an expansion in volume.
- The increased vibration results in greater distances between particles, causing the substance to occupy more space.
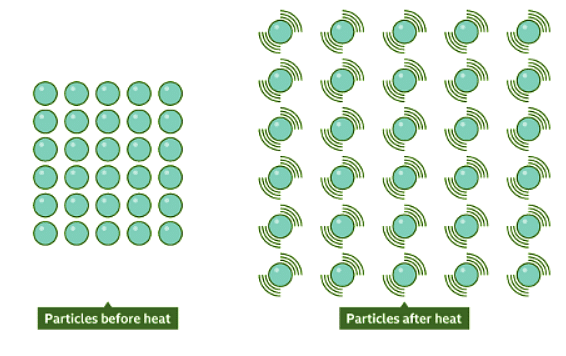
Contracting Matter
- Contrary to expanding matter, contracting matter involves a decrease in volume. This contraction happens when the particles in a substance move closer together.
- For example, when a substance contracts, its volume decreases. This contraction effect is particularly noticeable in objects like solids and liquids.
Changing density in an expanding object
- When an object expands, its particles move farther apart, increasing its volume. Since the number of particles remains the same, the mass of the object doesn't change.
- Therefore, the density decreases because density is mass divided by volume, and with the volume increasing while mass stays constant, the resulting ratio yields a lower density.
Changing density in a contracting object
- When an object contracts, its mass stays constant but its volume decreases. This leads to an increase in density because the same mass is now confined in a smaller space.
FAQs on Density of Solids, Liquids and Gases - Year 7
| 1. How do you calculate the density of a substance? |  |
Ans. Density is calculated by dividing the mass of an object by its volume. The formula for density is: Density = Mass/Volume.
| 2. How can you measure the density of an irregular shape? |  |
Ans. The density of an irregular shape can be measured by first finding the volume using water displacement method and then dividing the mass by the volume.
| 3. What is the difference between the density of solids, liquids, and gases? |  |
Ans. Solids have the highest density, followed by liquids, and then gases. This is due to the particles being more closely packed in solids compared to liquids and gases.
| 4. How does temperature affect the density of a substance? |  |
Ans. Generally, as the temperature of a substance increases, its density decreases. This is because the particles in the substance gain more kinetic energy and spread out, causing the density to decrease.
| 5. Can a substance expand or contract and still maintain the same density? |  |
Ans. Yes, a substance can expand or contract and still maintain the same density. This is because density is dependent on the mass and volume of a substance, and as long as these two properties change proportionally, the density remains constant.
Related Searches













