Year 7 Exam > Year 7 Notes > Chromatography
Chromatography - Year 7 PDF Download
| Table of contents |

|
| Introduction |

|
| Separating Dissolved Solids using Chromatography |

|
| Analysing chromatograms |

|
| Chromatogram |

|
Introduction
- Chromatography is a method used to separate mixtures of soluble substances.
- This technique is commonly applied to separate colored substances like food colorings, inks, dyes, or plant pigments.
- A chromatogram is the paper displaying results post-chromatography. By analyzing the chromatogram, one can draw conclusions about the pigments present in the mixture. It helps in matching known pigments with those in the mixture.
- On a chromatogram, a single spot indicates purity of the substance, while an impure substance results in two or more spots.
Separating Dissolved Solids using Chromatography
- An explanation on how chromatography is utilized to separate mixtures of soluble substances.
- When a solution contains multiple dissolved substances, chromatography can be used to separate them.
- This is particularly effective for separating different colored pigments, such as those in ink or food coloring.
- Chromatography works because some pigments are more soluble in water than others. Pigments with higher solubility travel further up the paper.
- Two methods of chromatography include:
- Standard chromatography
- Radial chromatography
Standard Chromatography
- Standard chromatography involves analyzing chromatograms to draw conclusions about the pigments present in a mixture.
- In an example with four ink samples, the first sample on the left is a pigment mixture (blue and yellow), while the other three are pure pigments (blue, pink, and yellow).

Method:
Steps for Standard Chromatography:
- Gather Materials: You will need a pencil, a ruler, a piece of chromatography paper, a colored mixture (e.g., a food dye), and a suitable solvent (e.g., water).
- Prepare the Chromatography Paper:
- Draw a horizontal line about 1 cm above the bottom of the chromatography paper using a pencil and ruler.
- Place a small spot of the colored mixture you are investigating onto the pencil line and let it dry.
- Optionally, add spots of pure colored pigments next to the mixture spot for comparison.
- Set Up the Experiment:
- Gently lower the piece of chromatography paper into a beaker containing a small amount of solvent. Ensure the solvent level is below the pencil line and does not touch the spots.
- You might need to hang the paper from a pencil or stick resting across the top of the beaker to keep it in place.
- Observe the Process:
- Watch as the solvent rises up the chromatography paper, reaching the spots on the pencil line.
- As the solvent continues to rise, it will dissolve the pigments in the spots and cause them to move up the paper.
- The colored mixture will start to separate into different spots or ovals positioned vertically above their original locations.
Radial Chromatography
- Radial chromatography is a method used to separate pigments in ink. This technique involves adding water onto a spot of ink in the center of a piece of filter paper. As the water spreads outwards, the pigments in the ink separate, showing different colors. Unlike standard chromatography where substances move upwards, in radial chromatography, they move outwards from the center.
- The process involves adding water onto a spot of ink in the center of a piece of filter paper.
- Pigments separate as they move outwards from the center.
Question for ChromatographyTry yourself: How does chromatography separate mixtures of soluble substances?View Solution
Analysing chromatograms
- Chromatography and its Purpose:
- Chromatography is frequently utilized to compare an unidentified mixture with pure pigments to determine the presence of those pigments.
- Standard Reference Pigments:
- Standard reference pigments, whose identities are known prior to a chromatography experiment, are utilized to ascertain their presence in the mixture under investigation.
- These pigments help in identifying the pigments present in the mixture being analyzed.
- Chromatogram Analysis:
- The chromatogram, the paper produced after a chromatography experiment, reveals insights about the pigments present in the mixture.
- Analyzing the chromatogram allows for conclusions to be drawn about the pigments within the mixture.
Chromatogram
A chromatogram is a crucial output of chromatography, revealing details about the pigments present in a mixture.
Interpreting Chromatograms
- After chromatography, a pure substance will exhibit only one spot on the chromatogram, indicating its purity.
- For instance, in a chromatogram displaying five pigment samples, the pure yellow pigment is visibly distinct, showcasing purity.
Following the process of chromatography, a pure substance will leave just one spot on the chromatogram. The photo demonstrates that the yellow pigment on the left is a pure pigment.
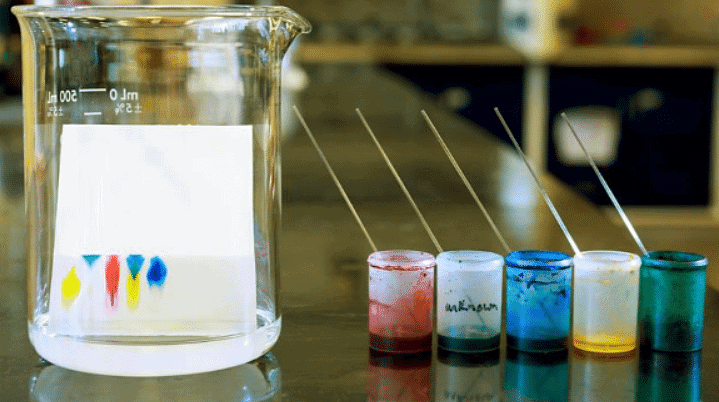
Deciphering Mixtures
- A mixture will separate into more than one spot on a chromatogram, typically forming a vertical column.
- The height of each spot signifies the substance's solubility, with higher spots indicating higher solubility.
- By comparing the color and height of spots, one can pinpoint the specific pigments present in the original mixture.
Using this information, we can work out that in the image above:
- The mixture in the fourth position comprises a blend of yellow and blue pigments.
- The blue pigment demonstrates higher solubility in the solvent, hence its position.
- The fourth substance includes the same yellow pigment as the substance on the left of the chromatogram, along with the identical blue pigment as the one in the second position from the left.
FAQs on Chromatography - Year 7
| 1. How does chromatography separate dissolved solids? |  |
Ans. Chromatography separates dissolved solids based on their different affinities for the stationary phase and mobile phase. As the mixture is passed through the stationary phase, the components in the mixture will interact differently with the stationary phase, causing them to separate.
| 2. What is a chromatogram? |  |
Ans. A chromatogram is a visual representation of the separation of components in a mixture during chromatography. It shows the retention time of each component, which is the time it takes for the component to travel through the column and be detected.
| 3. How can chromatograms be analyzed? |  |
Ans. Chromatograms can be analyzed by measuring the peak areas, heights, and retention times of the components in the mixture. This data can then be used to identify and quantify the components present in the sample.
| 4. What is the purpose of analyzing chromatograms? |  |
Ans. Analyzing chromatograms allows for the identification and quantification of components in a mixture. This information is crucial in various fields such as chemistry, biology, and forensics for determining the composition of samples.
| 5. Why is chromatography commonly used in scientific research? |  |
Ans. Chromatography is commonly used in scientific research because it is a versatile and powerful technique for separating and analyzing mixtures. It allows for the identification of components in complex samples and is essential for various scientific disciplines.
Related Searches













