Density - Year 7 PDF Download
Key points
 Image caption
Image caption- The density of an object or substance is defined as its mass divided by its volume.
- Density is a measure of how closely packed particles are within a substance. When particles are closely packed, the substance has a higher density compared to when particles are spread out.
- Mass refers to the amount of matter contained in an object. Unlike weight, which is influenced by gravity, an object's mass remains constant regardless of its location.
- Volume represents the space occupied by a three-dimensional shape, typically measured in cubic units such as cm³, mm³, or m³. It can also be referred to as capacity.
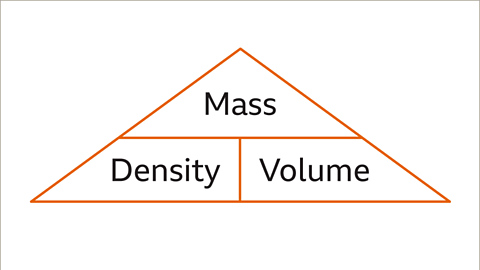
- The formula for calculating density is Density = Mass ÷ Volume.
Understanding Density
- The Concept of Density
- Density is a measure of how much mass is contained in a given volume.
- When the mass of an object is packed into a small space, it is considered dense.
- For instance, a dense object feels heavier because its particles are closely packed together.
- Units of Density
- Density is typically expressed in units like g/cm³, where mass is measured in grams and volume in cubic centimeters.
- The formula Density = Mass ÷ Volume allows comparison of densities across objects of varying sizes.
How to compare solids and liquids
- Substances comparison through density:
- Different densities of liquids result in floating of the least dense liquid on top.
- Varying densities exist among different types of wood. Hardwood, being denser, feels heavier compared to softwood.
- Denser objects occupy less space than the same mass of a less dense material. For instance, a metric tonne of feathers takes up more volume than a metric tonne of bricks.
Density and Volume Relationship
- If two liquids with different densities are mixed, the less dense liquid will float to the top.
- Different types of wood exhibit varying densities. Hardwood, being denser, feels heavier compared to softwood.
- Objects with higher density occupy less space than the same mass of a less dense material. For instance, a metric tonne of feathers would occupy significantly more volume than a metric tonne of bricks.
Metric Units in Density Comparison
- Density plays a crucial role in understanding volume differences between materials.
- In the metric system, units like meter, centimeter, millimeter, and kilometer are used to measure length and distance.
- For example, a tonne of feathers would occupy more space than a tonne of bricks due to the differing densities of the materials.
Understanding Density and Liquid Mixing
When different liquids are mixed together in a container, their densities determine their behavior.
- Each liquid's density affects the outcome based on its specific weight.
- Visible effects of density are clearer when liquids have distinct colors.
Examples
Observation of Density Effects
Observing the interaction between different liquids based on their densities:
- When various liquids are combined, such as vegetable oil, water, washing up liquid, maple syrup, and honey, their densities lead to stratification.
- The densest liquid, honey, settles at the bottom, while the least dense, vegetable oil, rises to the top.
- Distinct colors aid in visualizing the impact of density variations.
Visual Representation
Using an image illustrating the density effects in liquid mixing:
- Layering liquids with different densities in a container, from honey to vegetable oil, demonstrates how they stratify based on density.
 |  |  |
Understanding Density
When it comes to density, it's all about how tightly packed the matter in an object is. Let's dive into the concept with some examples:
Metal Bars and Density
- Solids, like metal bars, can have different densities despite having the same volume. For instance, if we compare two metal bars, one made of aluminum and the other of copper, although they are of the same size, the copper bar would feel heavier because it has a greater density. This is due to the atoms being more tightly packed in the copper bar.
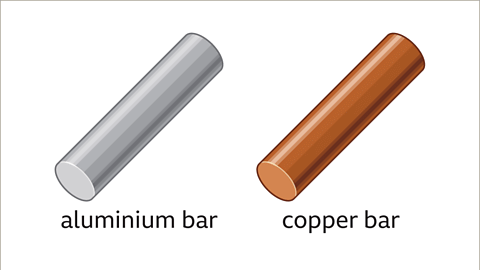
Density Comparison
- For example, the density of aluminum is 2.70g/cm³, while that of copper is 8.95g/cm³. This means that copper is denser than aluminum, and if we have bars of the same size made from these metals, the copper bar would be heavier.
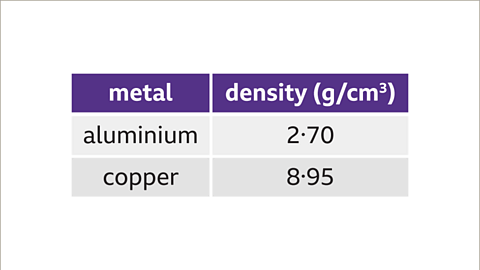
Understanding Density Formula
- The density of an object is calculated by dividing its mass by its volume. Density is usually measured in units like g/cm³. When comparing the densities of different substances, it's crucial to ensure they are in the same units for an accurate comparison.
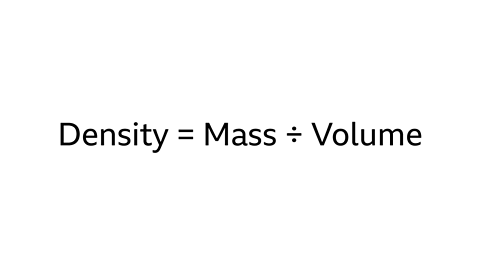
Slide 1 of 6

- An image shows three cylindrical measuring beakers of different sizes.
- The small beaker holds a blue liquid, the medium beaker contains red liquid, and the large beaker has green liquid.
- When different liquids are mixed together, their densities influence the outcome based on each liquid's density.
- The physical effects of density are more visible when liquids of different colors are used.
Question
Back to top
How to work out density
- Density is the mass per unit volume. It represents the amount of space occupied by a 3D shape, measured in cubic units like cm³, mm³, and m³. This concept is sometimes also known as capacity.
- Calculating Density: There are various methods to determine density. For solid, liquid, or gas, a common approach is to divide its mass (measured in grams, g) by its volume in cubic centimeters (cm³). For larger objects, density is calculated by dividing the mass in kilograms (kg) by the volume in cubic meters. The formula for density is Density = Mass ÷ Volume. The units for density will depend on the units used for mass and volume.
- Example: Consider a 500g object with a volume of 250cm³. To find its density, you would divide 500g by 250cm³, resulting in a density of 2g/cm³.
Volume
- Volume refers to the space occupied by a 3D shape, typically measured in cubic units like cm³, mm³, and m³. It can also be known as capacity.
- Calculating volume involves determining the amount of space a shape occupies in 3-dimensional space.
- For instance, when measuring a cube, you would calculate its volume by multiplying the length, width, and height.
- Understanding volume is crucial in various fields like architecture, physics, and engineering.
Density Calculation
- Density is a measure of how much mass is contained in a given volume.
- To calculate density, divide the mass (in kilograms or grams) by the volume (in cubic centimeters or cubic meters).
- The formula for density is Density = Mass ÷ Volume.
- For example, the density of water is approximately 1 g/cm³, which means 1 gram of water occupies 1 cubic centimeter of space.
Density Calculation
Mass is the measure of the amount of matter an object contains, typically expressed in kilograms (kg).
- If the object is large, density is computed by dividing its mass in kilograms by its volume in cubic meters: Density = Mass ÷ Volume.
- The units for density are derived from the units provided for mass and volume.
Example
Calculate the density of different types of metals using the mass and volume of each object for comparison.
- For instance, to compare the densities of two different metals like a lead pipe and an iron bar, the mass and volume need to be considered. The lead pipe has a mass of 2268 grams and a volume of 200 cm³, while the iron bar has a mass of 3537 grams and a volume of 450 cm³.
| Type of Metal | Mass (grams) | Volume (cm³) |
|---|---|---|
| Lead | 2268 | 200 |
| Iron | 3537 | 450 |
Density and Mass Relationship in Metals
To determine the density of different metals, the formula Density = Mass ÷ Volume is essential.
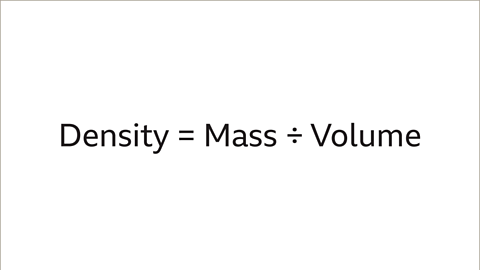
A mnemonic method involves using the Mass, Density, Volume triangle to remember the density formula.
1. Lead Pipe Density: 2268 grams ÷ 200 cm³ = 11.34 g/cm³.
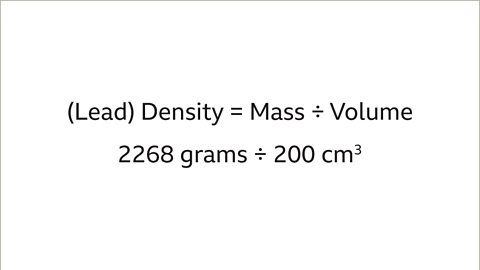
2. Iron Bar Density: 3537 grams ÷ 450 cm³ = 7.86 g/cm³.
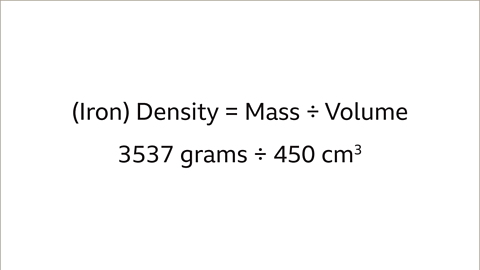
Lead has a higher density than iron. Lead's density is 11.34 g/cm³ compared to iron's 7.86 g/cm³. If blocks of lead and iron were the same size, the lead block would be heavier due to its greater mass.

Slide 1 of 7
An image of two identical cylinders. Written below the first cylinder: lead pipe. Written below the second cylinder: iron bar. The lead cylinder is colored a dark metallic grey. The iron cylinder is colored a lighter metallic grey.
Question
Calculate the density of these different types of metals.
Paraphrased Information
- Identical Cylinders
Two cylinders are depicted, one labeled as a lead pipe and the other as an iron bar. The lead cylinder is dark metallic grey, while the iron cylinder is a lighter metallic grey.
- Calculation of Density
To determine the density of these metals, we need to consider their mass and volume. Density is calculated by dividing the mass of the substance by its volume. For example, if the lead cylinder has a mass of 100 grams and a volume of 50 cm³, its density would be 2 g/cm³.
- Importance of Density
Density is a crucial property of materials as it helps in identifying and distinguishing different substances. Materials with higher density are heavier for their size compared to those with lower density.
- Real-World Applications
Understanding density is vital in various fields such as engineering, chemistry, and materials science. For instance, in architecture, the density of building materials affects the structural integrity of a construction.
Practise your understanding of density
Quiz
Practise your comprehension of density application in mathematics with this quiz. Have a pen and paper ready to assist with your responses.
Game - Divided Islands
 |  |
- Density is a measure of how much mass is contained in a given volume.
- It is calculated by dividing an object's mass by its volume.
- For example, a material with high density has more mass packed into a specific volume compared to a material with low density.
- Understanding density is crucial in various fields like engineering, physics, and chemistry.
- For instance, in shipbuilding, engineers consider the density of materials to ensure the ship floats correctly.
- In mathematics, density can be used to solve problems involving mass and volume.
- For example, when determining the density of a substance, one needs to measure its mass and volume accurately.
- Divide the mass by the volume to obtain the density of the substance.
- The standard unit of density in the International System of Units (SI) is kilograms per cubic meter (kg/m³).
- Other common units include grams per cubic centimeter (g/cm³) and pounds per cubic inch (lb/in³).
Density Basics
Real-World Applications
Mathematical Calculations
Common Units
Back to top



















