Notes: Things We Make and Do | EVS & Pedagogy Paper 1 for CTET & TET Exams - CTET & State TET PDF Download
How We Make Things?
Everything in the universe is made up of elements and matter. These elements and matter are formed from molecules, which are in turn made up of atoms. Humans rely on the resources available on Earth to meet their needs. We modify and reshape these resources to make them useful for various purposes.
Things We Know
Almost everything we use around us is a product of science and technology. Everyday items like fans, wheels, vehicles, clothes, paper, electricity, and radios are all results of scientific advancements and technological innovations.
What is Matter?
Everything in the universe, including air, food, stones, clouds, stars, plants, water, and animals, is made up of matter. Matter is the material that composes all these things. 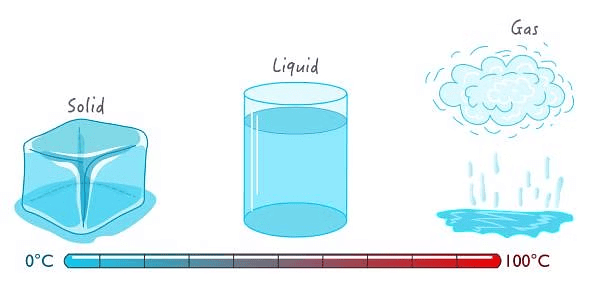
Characteristics of Matter
- Particles of matter have space between them. When we make drinks like nimbu paani (lemonade) or coffee, the particles of one type of matter fit into the spaces between the particles of another type. This mixing happens because of a process called diffusion.
- Particles of matter are constantly moving due to the presence of kinetic energy. When the temperature increases, the particles move faster.
- Particles of matter attract each other because of a force that pulls them together. This force keeps the particles close to each other, but its strength varies depending on the type of matter.
States of Matter
Matter exists in three states:solid, liquid, and gas. The differences in the characteristics of particles in these states determine the behavior of matter.
1. Solid State: Solid matter has a definite shape, distinct boundaries, and a fixed volume. Even when an external force is applied, solids tend to maintain their shape. While they can break under force, it is difficult to change their shape, making them rigid. Examples include bricks, stones, and ice.
2. Liquid State: Liquids do not have a fixed shape but have a fixed volume. They take the shape of the container they are in. Liquids can flow and change shape, which makes them fluid. Examples include water, milk, and oil.
3. Gaseous State: In the gaseous state, both the volume and shape are not fixed. An example of this is air.
Water is unique because it can exist in all three states: solid (ice), liquid (water), and gas (water vapor).
Classification of Matter
Matter can be classified into elements, compounds, and mixtures.
1. Elements
Elements are substances that contain only one kind of atom or molecule. Each atom of an element has a specific mass, known as atomic mass. Elements can be further classified into metals and non-metals.
Metals
- Shining Surface: Metals have a shiny surface when in their pure form.
- Malleability: Metals can be hammered or rolled into thin sheets.
- Ductility: Metals can be drawn into thin wires.
- Good Conductors: Metals are excellent conductors of heat and electricity.
Some common metals include: Silver, gold, copper, lead, etc.
Non-Metals
- Non-metals can be solids or gases, with the exception of bromine, which is a liquid.
- Non-metals are generally poor conductors of heat.
- Some non-metals, like iodine, have a shiny surface.
Examples of Non-Metals: Carbon, sulfur, iodine, oxygen, hydrogen, etc.
Metalloids: Some elements exhibit properties of both metals and non-metals and are called metalloids. Examples include boron, silicon, and germanium.
2. Compounds
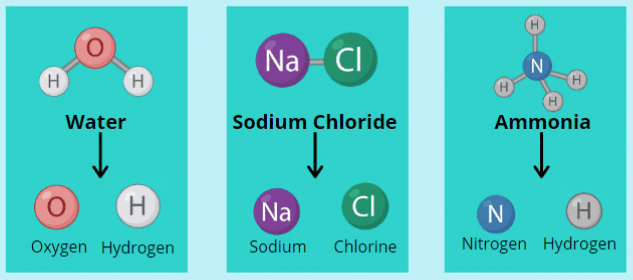 Compounds Compounds are substances made up of two or more elements chemically combined together. For example, water (H2O) is a compound made of two hydrogen atoms and one oxygen atom.
Compounds Compounds are substances made up of two or more elements chemically combined together. For example, water (H2O) is a compound made of two hydrogen atoms and one oxygen atom.
3. Mixtures
Mixtures can contain a combination of elements and/or compounds. For instance, air is a mixture of oxygen, nitrogen, carbon dioxide, and water vapor.
Types of Mixtures:
Mixtures can be classified into two types based on the nature of their components:
- Homogeneous Mixture: A mixture with a uniform composition throughout, such as salt dissolved in water or sugar dissolved in water.
- Heterogeneous Mixture: A mixture with physically distinct parts and non-uniform composition, such as a mixture of sodium chloride, iron filings, oil, and water.
Solution
A solution is a homogeneous mixture of two or more substances. In a solution, the components are uniformly distributed at the particle level. For example, lemonade tastes the same throughout because it is a solution.
A solution consists of a solvent and a solute. The solvent is the substance that dissolves the other component (usually in a larger amount), while the solute is the substance that is dissolved in the solvent. 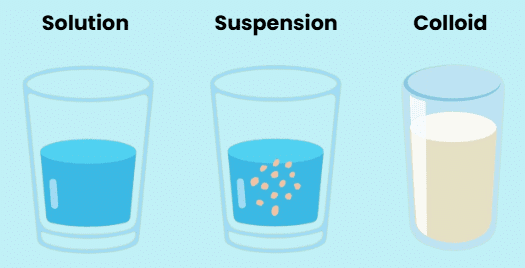
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium. The particles in a suspension are visible to the naked eye. An example of a suspension is muddy water, where the soil particles are suspended in water.
Colloidal Solution
A colloidal solution consists of particles that are uniformly spread throughout the solution. Although the mixture appears homogeneous due to the relatively small size of the particles, it is actually a heterogeneous mixture. An example of a colloidal solution is milk. Colloids have the ability to scatter a beam of visible light, a phenomenon known as Tyndall's effect.
Alloy
An alloy is a mixture of two or more metals or a metal and a non-metal. Alloys cannot be separated into their individual components by physical methods. For example, brass is an alloy made up of approximately 30% zinc and 70% copper.
Acids and Bases
Acids are substances that taste sour and turn blue litmus paper red. They have a pH less than 7 on the pH scale, which ranges from 1 to 14. The pH scale measures the acidity and basicity of a substance.
Acids and Their Related Matters
| Acid | Related Matter |
| Citric acid | Lemon, orange, etc. |
| Acetic acid | Vinegar |
| Lactic acid | Curd |
| Formic acid | Red ant |
| Carbonic acid | Cold drinks |
| Tartaric acid | Tamarind |
| Oxalic acid | Tomato |
| Malic acid | Apple |
| Uric acid | Urine |
Bases
- Bases taste bitter and turn red litmus paper blue. They have a pH greater than 7.
- Natural indicators for acids and bases include litmus and turmeric.
- Synthetic indicators include methyl orange and phenolphthalein.
Physical and Chemical Changes
Physical changes are typically related to the physical states of matter. Examples of physical changes include:
- Crushing a can
- Melting of an ice cube
- Breaking a glass
- Shredding paper
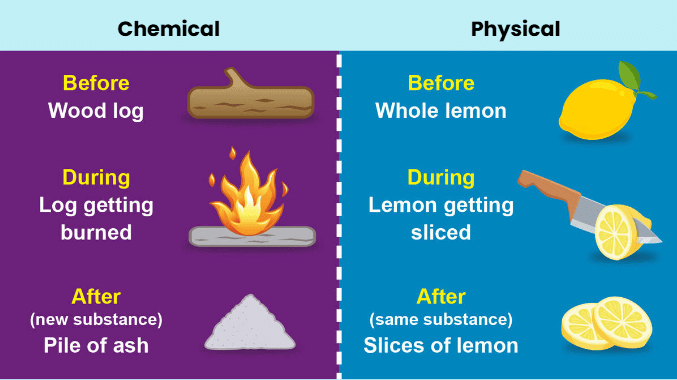
Chemical changes, on the other hand, occur at the molecular level and involve chemical reactions where atomic bonds are broken or formed. Examples of chemical changes include:
- Rusting of iron
- Combustion of wood
- Cooking an egg
- Baking a cake
- Rotting bananas
- Milk becoming sour
Separation Techniques
There are various techniques used for separating different mixtures. Here are some methods for separating solid components:
Separation of Solid Components
Solid components can be separated using the following techniques:
- Manual Separation: This method involves separating materials by hand. For example, separating concrete from rice or pulses.
- Threshing: Threshing is the process of separating the edible part or grain from the stalk and husk that surrounds it. This process is commonly used during grain preparation after harvesting. Nowadays, threshing machines are often used to make this process more efficient.
- Winnowing: Winnowing involves blowing a current of air through grain to separate the chaff (the outer covering) from the grain. This process is typically done after threshing.
 Winnowing
Winnowing - Filtration: Filtration is used to separate fine matter present along with grain. A fine net is used to filter out the unwanted material. For example, filtration can be used to separate sand from concrete or to filter flour.
- Magnetic Separation: This technique uses a magnet to separate magnetic materials such as iron, cobalt, nickel, and steel from non-magnetic materials.
Separation of Solid and Liquid
Solid and liquid components can be separated using the following techniques:
- Sedimentation: Sedimentation involves allowing a mixture of liquid and suspended solid particles to sit undisturbed in a container. Over time, the solid particles settle to the bottom of the container due to the force of gravity.
- Decantation: Decantation is the process of transferring the liquid from one vessel to another without disturbing the settled solid or the liquid mixture. This technique is used after sedimentation, where the surface liquid is carefully poured off, leaving the solid or heavier liquid behind.
- Filtration: Filtration involves passing a mixture of solid and liquid through a net, filter paper, or membrane. The solid particles are trapped in the net or membrane while the liquid passes through easily.
 Filtration
Filtration - Evaporation: Evaporation involves heating a mixture of solid and liquid to evaporate the liquid, leaving the solid behind as residue. For example, this method can be used to separate sugar and salt from a solution.
- Centrifugation: Centrifugation involves rotating a mixture at high speed, causing the solid particles to settle at the bottom rapidly due to centrifugal force. This method is commonly used to separate cream from milk or to make butter from milk.
- Distillation: Distillation involves heating a mixture of solid and liquid to form vapors, which are then collected and condensed in a specific container. This technique is used to separate desired components from the mixture.
Separation of Liquid from Liquid Mixture
Liquids from liquid mixtures can be separated using the following techniques:
- Separation Cone: A separation cone is used to separate two immiscible liquids (liquids that do not mix). When two immiscible liquids are present in the cone, they form distinct layers. The lower layer can be drained from the cone, leaving only the upper layer liquid.
- Fractional Distillation: In fractional distillation, liquids are heated at different temperatures, and the vapors are collected and condensed in separate containers for each temperature. This method is used to separate components from mixtures, such as in the case of crude petroleum, where different products like petrol, diesel, and kerosene are obtained by heating at various temperatures.
Force and Friction
- Force is any push or pull exerted on an object, causing a change in its motion or position. It can result from gravity, magnetism, electricity, or any factor that accelerates a mass. The SI unit of force is Newton (N), represented by the symbol "F."
- Force has both magnitude and direction. Friction, on the other hand, is a force that opposes motion due to a push or pull. It acts in the opposite direction of the driving force. Examples of friction include:
- Applying brakes to stop a vehicle.
- Walking on the ground without slipping.
- Writing on paper, where friction occurs between the pen tip and the paper.
Things We Do
Economic activities involve the production, distribution, and consumption of goods and services.
Examples of economic activities include producing goods and providing services.
Economic Activities
Economic activities can be classified into three groups:
1. Primary Activities
- Primary activities involve exploiting naturally occurring resources.

- They are called primary because they provide the raw materials for all other economic activities.
- Examples include agriculture, fishing, forestry, and animal husbandry.
2. Secondary Activities
- Secondary activities involve processing and manufacturing the natural products from primary activities into finished goods.
- These activities are often associated with industries and manufacturing.
3. Tertiary Activities
- Tertiary activities do not produce goods directly but support the production process.
- Examples include transportation, communication, banking, storage, and trade.
Agriculture
1. Pastoralism
- Focuses on raising livestock, such as camels, goats, cattle, yaks, and sheep.
2. Shifting Agriculture
- Involves clearing a small area of forest by cutting and burning trees.
- Used for growing crops for a few years until the soil becomes less fertile.
- Afterward, the area is abandoned and not used for agriculture.
3. Jhum Cultivation  Jhum Cultivation
Jhum Cultivation
- A form of shifting agriculture also known as slash and burn cultivation.
- Land is cleared by felling trees and burning remaining vegetation.
- Farming is done on the burnt field, as burning adds nutrients to the soil.
- This method is mainly practiced in the Northeastern states of India.
- It poses environmental challenges due to deforestation.
4. Subsistence Farming
- Aims to meet the food needs of the family and local community.
5. Intensive Farming
- Focuses on commercial production of crops.
- The primary goal is to generate profit.
6. Contour Farming
- Practiced in mountain regions to conserve rainwater and reduce soil erosion.
- Involves farming along contour lines around the hill, rather than up and down.
Specific Types of Agriculture
- Viticulture: Commercial production of grapevines.
- Pisciculture: Commercial production of fish.
- Sericulture: Production of silk and rearing of silkworms.
- Horticulture: Cultivation of fruits, flowers, and vegetables.
- Apiculture: Rearing bees for honey production.
- Silviculture: Growing and cultivation of trees.
- Floriculture: Cultivation of flowers.
- Arboriculture: Cultivation of trees and shrubs.
- Mariculture: Production of marine animals.
- Olericulture: Commercial cultivation of vegetables grown on creepers.
- Vermiculture: Rearing of earthworms to enhance soil fertility.
Cloth
Some household items like bed sheets, curtains, tablecloths, blankets, and clothes are made from different types of cloth. These cloths are produced from cotton, wool, silk, and other fibers. Some fibers, such as cotton, jute, silk, and wool, are obtained from plants and animals and are called natural fibers. Other types of fibers are made from synthetic materials. Examples of synthetic fibers include polyester, nylon, and acrylic.
Cotton
- Cotton plants thrive in black soil and a warm climate.
- The fruits of the cotton plant, known as cotton bolls, are about the size of a lemon.
- When the bolls mature, they burst open, revealing the seeds covered with cotton fiber.
- Cotton is primarily used in the cloth industry.
- It is also used to stuff pillows and mattresses and as a bandage in hospitals.

Jute
- Jute is obtained from the stem of the jute plant, which is cultivated during the rainy season.
- In India, jute is grown in West Bengal, Bihar, and Assam.
- The jute plant is harvested when it is at the flowering stage.
- The stems of the harvested plants are immersed in water for a few days to separate the roots and fibers by hand.
- Jute is primarily used for making jute bags, chair coverings, carpets, and area rugs.
Wool
- Sheep wool is the most commonly available type of wool in India.
- The process of converting wool into fiber involves several steps: shearing, scouring, sorting, cleaning of burrs, dyeing, straightening, combing, and finally rolling into yarns.
Synthetic Fibres
Rayon
- Rayon is synthesized from wood pulp and resembles silk, which is why it is also known as artificial silk.
- It is much cheaper than silk.
Nylon
- Nylon is synthesized from coal, water, and air.
- It is known for its strength and also resembles silk.
- Nylon is used to make various items such as clothes, ropes, brushes, curtains, and bags.
Polyester
- Polyester fibers are extremely strong, durable, resistant, and do not wrinkle easily.
- They do not absorb water, allowing them to dry quickly.
- Polyester is used to make garments, bed sheets, and other products.
Acrylic
- Acrylic is a lightweight, soft, and warm synthetic fiber.
- Acrylic fiber is commonly used for making sweaters, tracksuits, furnishing fabrics, and carpets.
Fertilizers
- Fertilizers are materials used to provide plant nutrients that are deficient in soils.
- Many fertilizers are extracted and purified from natural deposits in the Earth.
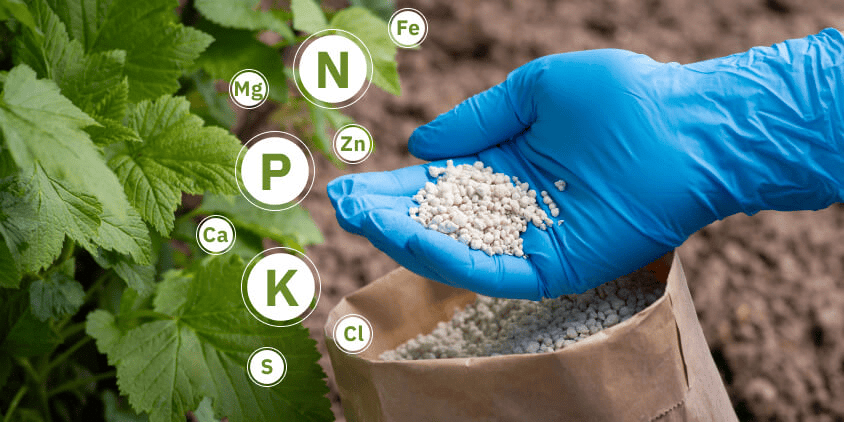
- Materials such as Sul-Po-Mag, muriate of potash, and triple super phosphate are produced from naturally occurring minerals.
- Some materials, such as urea and ammonium nitrate, are synthetic but provide plants with the same nutrients found naturally in the soil.
Nitrogen Based Fertilizers
- Nitrogen plays a vital role in the protein formation process of plants.
- Nitrogen-based fertilizers are made from ammonia.
Phosphorus Based Fertilizers
- These fertilizers contain phosphorus in absorbable form and are used for crops like fruit trees, potatoes, and chillies.
Potassium Based Fertilizers
- Potassic fertilizers contain potassium in absorbed form.
- They are water soluble and readily available to plants.
Plastic
Plastic is a synthetic material made from a wide range of organic polymers such as polyethylene, PVC, nylon, etc., that can be molded into different shapes.
Plastic can be divided into two major categories:
Thermosetting Plastics
- Once cooled and hardened, these plastics retain their shape and cannot return to their original form.
- They are hard and durable.
- Thermosets can be used for auto parts, aircraft parts, and tires.
Thermoplastics
- These are less rigid than thermosets.
- Thermoplastics can soften upon heating and return to their original form.
- They are easily molded and extruded into films, fibers, and packaging.
Glass
Glass is a solid material that is usually transparent and breaks easily. It is formed by fusing sand, sodium carbonate, and calcium carbonate under extreme heat. Glass is used for the construction of windows, eyeglasses, bottles, etc.
Glass is made by melting together several minerals at very high temperatures. The main ingredient is silica in the form of sand, which is combined with soda ash and limestone and melted in a furnace at high temperatures.
Magnet
A magnet is a piece of metal with a strong attraction to another metal object. The attraction a magnet produces is called a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other objects, such as iron.
Types of Magnets
Natural Magnet
- A natural magnet is one that occurs naturally in nature.
- All natural magnets are permanent magnets, meaning they never lose their magnetic power.
Artificial Magnet
- When magnets are man-made, they are called artificial magnets.
- These types of magnets often have extra-strong magnetic power.
- Artificial magnets can be either temporary or permanent.
Festivals in India
- A festival is a community celebration based on its unique aspects, often linked to religion or traditions, and usually observed as a local or national holiday.
- India is renowned globally for its rich tapestry of cultural and traditional festivals, reflecting its diverse cultures and religions.
- Throughout the year, one can witness vibrant festival celebrations in India, a secular nation embracing diversity in religions, languages, cultures, and castes.
Things We Make
- The crafts of India are diverse, rich in history and religion. Throughout centuries, crafts have been embedded as a culture and tradition within rural communities.
Painting
The tradition of painting has been carried on the Indian subcontinent since ancient times. Different types of paintings are given below.
Chitra Kathi Painting
- Origin: Maharashtra
- Characteristics: Depicts stories using only natural colors.
Kalamkari Painting  Kalamkari Painting
Kalamkari Painting
- Origin: Andhra Pradesh
- Characteristics: Uses natural colors, often done on cloth through block printing.
Madhubani Painting
- Origin: Bihar
- Characteristics: Depicts natural elements like leaves, flowers, animals, birds, and humans using natural colors. Special rice paste is used to enhance the painting.
Pata Painting
- Origin: Odisha
- Characteristics: Uses natural colors derived from minerals and vegetables.
Phad Painting
- Origin: Rajasthan
- Characteristics: Done on cloth with vibrant colors, often depicting religious themes.
Traditional Art
- Art that is an integral part of a community's culture, passed down through generations.
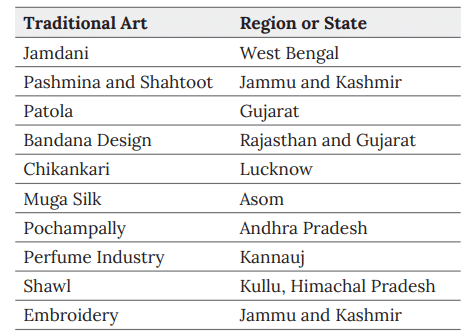 Some Traditional Art Forms
Some Traditional Art Forms
World Famous Pashmina
- Warmth: Pashmina shawls are incredibly warm, equivalent to six sweaters, yet very thin.
- Source: Wool is collected from goats at high altitudes (around 5000 meters) where temperatures can drop to −40°C. These goats grow a fine coat of hair for warmth, which is shed in summer.
- Quality: The fine hair is so thin that six strands are as thick as one human hair. This hair is too delicate for machine weaving.
- Craftsmanship: Pashmina shawls are handwoven in Kashmir, a process that is lengthy and labor-intensive. A plain shawl can take up to 250 hours to weave.
Languages
India is home to 22 major languages. Hindi and English are the most widely spoken across the country. Additionally, many states have their own official languages that are prevalent in specific regions.
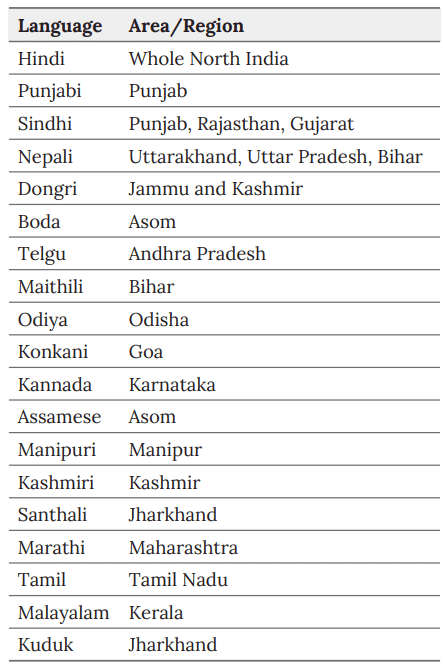 Different Language and Dialects of India
Different Language and Dialects of India
Tribes
A tribe is seen as a social group that existed before the formation of states or outside of them, either developmentally or historically. Tribes are distinct groups of people who rely on their land for sustenance, are largely self-sufficient, and are not fully integrated into the broader national society. 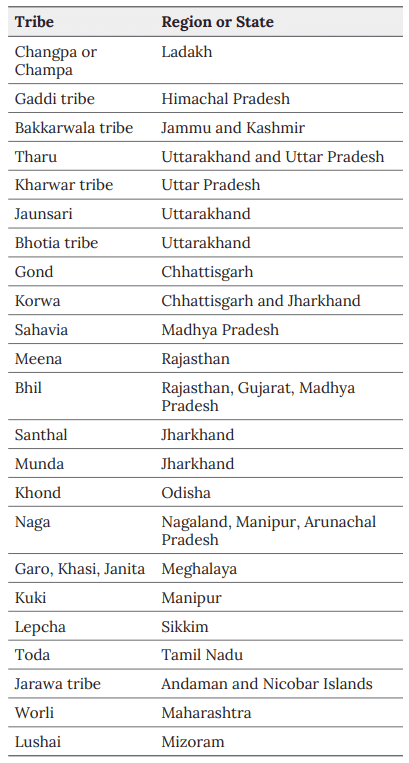 Famous Tribes of India
Famous Tribes of India
|
21 videos|139 docs|35 tests
|
FAQs on Notes: Things We Make and Do - EVS & Pedagogy Paper 1 for CTET & TET Exams - CTET & State TET
| 1. What are the different types of separation techniques used in science? |  |
| 2. How does friction affect motion in everyday activities? |  |
| 3. What are some common festivals celebrated in India? |  |
| 4. What are some simple things we can make at home? |  |
| 5. How do we use matter in our daily lives? |  |
















