Class 8 Exam > Class 8 Notes > Year 8 Chemistry (Cambridge) > Solutions and solubility
Solutions and solubility | Year 8 Chemistry (Cambridge) - Class 8 PDF Download
| Table of contents |

|
| Introduction to Solutions |

|
| Concentration of Solutions |

|
| Factors Affecting Solubility |

|
| Solubility and Saturated Solutions |

|
| Solubility Units |

|
| Factors Affecting Dissolving Rate |

|
Introduction to Solutions
- Solutions are homogeneous mixtures composed of a solvent and one or more solutes.
- The solvent is the substance in which the solute dissolves, forming a uniform mixture.

Concentration of Solutions
- Molarity (M): Molarity is defined as the number of moles of solute per liter of solution (mol/L).
- Example: A solution with 0.5 M concentration means there are 0.5 moles of solute in 1 liter of solution.
Question for Solutions and solubilityTry yourself: What is the definition of molarity?View Solution
Factors Affecting Solubility
- Temperature: Generally, solubility increases with temperature for solids and decreases for gases.
- Example: Sugar dissolves faster in hot tea compared to cold tea.
- Pressure: Affects mainly gases; higher pressure increases solubility.
- Example: Carbon dioxide is more soluble in soda under pressure.
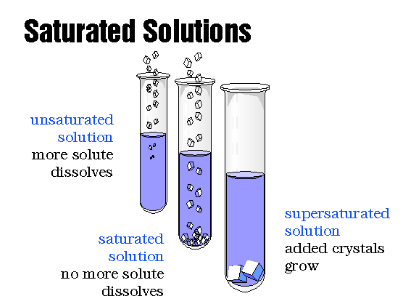
Solubility and Saturated Solutions
- Saturated Solution: Contains the maximum amount of solute that can dissolve at a given temperature.
- Example: A solution of sugar in water where no more sugar can dissolve at a specific temperature.
- Supersaturated Solution: Holds more solute than normally possible at a given temperature, achieved by careful cooling of a saturated solution.
- Example: Creating a supersaturated sugar solution by heating and then slowly cooling it.
Solubility Units
- Molality (m): Molality is the number of moles of solute per kilogram of solvent.
- Example: 1 molal (1 m) solution has 1 mole of solute dissolved in 1 kilogram of solvent.
Question for Solutions and solubilityTry yourself: What is a saturated solution?View Solution
Factors Affecting Dissolving Rate
- Stirring: Increases the rate by bringing fresh solvent into contact with the solute.
- Temperature: Generally, higher temperatures increase the rate of dissolution for solids in liquids.
- Particle Size: Smaller solute particles dissolve faster due to increased surface area.
Conclusion
Understanding solutions and solubility helps in various applications from chemistry labs to everyday life, such as cooking and cleaning.
The document Solutions and solubility | Year 8 Chemistry (Cambridge) - Class 8 is a part of the Class 8 Course Year 8 Chemistry (Cambridge).
All you need of Class 8 at this link: Class 8
|
6 videos|14 docs|4 tests
|
FAQs on Solutions and solubility - Year 8 Chemistry (Cambridge) - Class 8
| 1. What are some factors that can affect the rate at which a solute dissolves in a solvent? |  |
Ans. Factors that can affect the rate at which a solute dissolves in a solvent include temperature, stirring or agitation, surface area of the solute, and the nature of the solvent and solute.
| 2. How does temperature influence the solubility of a solute in a solvent? |  |
Ans. Generally, as temperature increases, the solubility of most solids in liquids also increases. However, the effect of temperature on solubility can vary depending on the specific solute and solvent involved.
| 3. What role does stirring play in the dissolving process of a solute in a solvent? |  |
Ans. Stirring or agitation can increase the rate of dissolving by ensuring that fresh solvent comes into contact with the solute, thus speeding up the process of solute particles being surrounded and dispersed by solvent particles.
| 4. How does the surface area of a solute affect its rate of dissolving in a solvent? |  |
Ans. A larger surface area of a solute allows for more contact points with the solvent, leading to a faster rate of dissolving compared to a solute with a smaller surface area.
| 5. Can you explain how the nature of the solvent and solute influence the solubility of a solution? |  |
Ans. The nature of the solvent and solute can affect solubility by determining the strength of the intermolecular forces between the solvent and solute particles. Compatible solvents and solutes with similar intermolecular forces are more likely to form a solution with higher solubility.
Related Searches















