Class 8 Exam > Class 8 Notes > Year 8 Chemistry (Cambridge) > Chemical Changes
Chemical Changes | Year 8 Chemistry (Cambridge) - Class 8 PDF Download
| Table of contents |

|
| Chemical Changes |

|
| Physical Changes |

|
| Types of Substances |

|
| Energy Changes in Reactions |

|
| Chemical Equations |

|
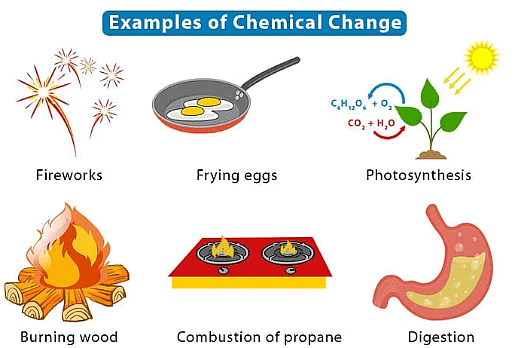
Chemical Changes
- Chemical changes involve the formation of new substances with different properties.
- Examples include:
- Burning wood, which produces ash and smoke.
- Rusting of iron, forming iron oxide.
- These changes are not easily reversible by physical means.
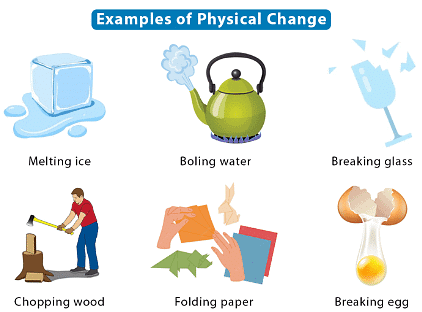
Physical Changes
- Physical changes alter the appearance or state of a substance without changing its composition.
- Examples include:
- Melting ice into water.
- Dissolving sugar in water.
- These changes are reversible by physical means.
Question for Chemical ChangesTry yourself: Which of the following is an example of a chemical change?View Solution
Types of Substances
Pure Substances
- Consist of only one type of particle.
- Examples:
- Carbon dioxide (CO2) is a pure substance.
- Gold (Au) is an element, also a pure substance.
- Can be compounds (like water) or elements (like oxygen).
Mixtures
- Combination of two or more substances that are not chemically combined.
- Examples:
- Salt dissolved in water.
- Air (a mixture of gases).
- Can be homogeneous (uniform composition) or heterogeneous (non-uniform composition).

Energy Changes in Reactions
Exothermic Reactions
- Release energy to the surroundings, usually as heat.
- Examples:
- Combustion reactions (e.g., burning fuels).
- Neutralization reactions.
Endothermic Reactions
- Absorb energy from the surroundings.
- Examples:
- Melting ice.
- Photosynthesis.
Chemical Equations
- Represent chemical reactions using symbols and formulas.
- Example:
- Combustion of methane: CH4 + 2O2 → CO2 + 2H2O
Measuring Changes in Reactions
- Use a thermometer to measure temperature changes.
- Important in determining whether a reaction is exothermic or endothermic.
Conclusion
Understanding chemical changes, types of substances, energy changes, and how to represent reactions with equations are essential in studying the properties of matter. These concepts help explain everyday phenomena and industrial processes involving substances and their transformations.
The document Chemical Changes | Year 8 Chemistry (Cambridge) - Class 8 is a part of the Class 8 Course Year 8 Chemistry (Cambridge).
All you need of Class 8 at this link: Class 8
|
6 videos|14 docs|4 tests
|
Related Searches















