Pollution and it's Types | General Awareness for SSC CGL PDF Download
| Table of contents |

|
| Environment |

|
| Atmospheric Pollution |

|
| Major Gaseous Air Pollutants |

|
| Consequences of Atmospheric Pollution |

|
| Stratospheric Pollution |

|
| Pollution Control Strategies |

|
Environment
The environment refers to the physical and biological world surrounding us, encompassing three main components:
- Abiotic or Non-living Components
- Biotic or Living Components
- Energy Components
Abiotic Components
Abiotic components, or abiotic factors, are the non-living chemical and physical elements of the environment that influence ecosystems. These include the atmosphere, hydrosphere, and lithosphere.
Atmosphere: The atmosphere provides essential gases such as oxygen, nitrogen, and carbon dioxide. Oxygen is crucial for respiration in plants and animals, while carbon dioxide is needed for photosynthesis. The atmosphere also carries water vapor, which leads to rainfall, protects life by blocking harmful UV rays, and helps regulate Earth's temperature. It consists of several layers:
- Troposphere: The lowest layer, extending up to 18 km, is turbulent and dusty, containing most of the Earth's air, water vapor, and clouds.
- Stratosphere: Above the troposphere, between 18-60 km, the temperature begins to rise. This layer includes the ozone layer, which absorbs about 99.5% of harmful UV radiation from the Sun, protecting living organisms. Ozone forms when UV light splits oxygen molecules into individual atoms, which then combine with other oxygen molecules.
- Mesosphere: Extending from 60-85 km above the stratosphere, the temperature decreases with altitude, reaching -100°C. This layer is where meteors burn up upon entering.
- Thermosphere: Above the mesosphere, reaching up to 640 km, temperatures can soar to 1500°C, but the low pressure means it doesn’t feel warm. The International Space Station orbits in this layer.
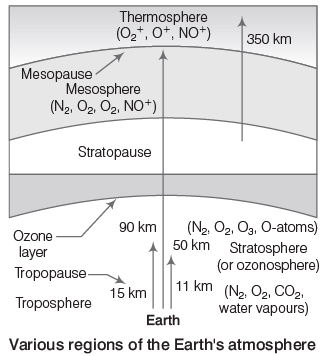
- Exosphere: The highest atmospheric layer, from 500-1600 km, contains ionized gases. Beyond this lies interstellar space.
Hydrosphere: This includes all the Earth's water sources such as oceans, rivers, lakes, and ponds. Ocean water is salty, with about 3.5% dissolved salts, making it unsuitable for drinking.
Lithosphere: The lithosphere is the solid, rocky outer layer of the Earth, comprising minerals and covering the planet from the summit of Mount Everest to the depths of the Mariana Trench.
Biosphere
The biosphere is the part of the abiotic components (lithosphere, hydrosphere, and atmosphere) where living organisms exist and interact with these elements.
Biotic Components
Biotic components are the living organisms that shape ecosystems. They include any living factor that impacts another organism, such as predators, prey, and plants that provide food. Each biotic factor requires energy and food to grow and function properly.
Atmospheric Pollution

Atmospheric pollution refers to the introduction of undesirable substances into the atmosphere that negatively impact plants, animals, and humans. This pollution can result from both natural processes and human activities. There are two main types of atmospheric pollution: tropospheric and stratospheric.
- Tropospheric Pollution: This can be either gaseous or particulate.
- Stratospheric Pollution: This affects the stratosphere and involves different pollutants.
Pollutants: Substances causing pollution are known as pollutants. They fall into two categories:
- Primary Pollutants: These remain in the environment in their original form, such as sulfur dioxide (SO₂) and nitrogen dioxide (NO₂).
- Secondary Pollutants: These are formed from the reaction of primary pollutants, including peroxyacetyl nitrate (PAN), ozone (O₃), and aldehydes.
Pollutants from the Steel/Iron Industry: The iron and steel industry contributes to air, water, and soil pollution. Air pollutants include carbon monoxide (CO), sulfur dioxide (SO₂), nitrogen oxides (NOₓ), carbon dioxide (CO₂), hydrogen sulfide (H₂S), PAN, lead (Pb), nickel (Ni), and cadmium (Cd). Water pollutants include organic matter, oils, metals, acids, sulfides, and sulfates.
Major Gaseous Air Pollutants
Sulfur Dioxide (SO₂): Highly toxic to both humans and plants, even at low concentrations. It can cause respiratory issues like bronchitis and asthma, as well as eye and throat irritation. In plants, it hampers chloroplast formation, leading to chlorosis. SO₂ also contributes to acid rain when it reacts with particulate matter and water to form sulfuric acid (H₂SO₄), which damages structures and materials.
Oxides of Nitrogen (NOₓ): This group includes nitric oxide (NO), a colorless, odorless gas, and nitrogen dioxide (NO₂), a brown gas with a pungent odor. NO₂ is toxic to tissues, causing leaf fall and reducing photosynthesis rates in plants. It also contributes to smog and can irritate eyes, as well as affect the liver and kidneys. NO₂ reacts with moisture to form nitric acid (HNO₃), a component of acid rain.
Carbon Monoxide (CO): This gas is highly poisonous as it forms a stable complex with hemoglobin in the blood, reducing oxygen transport to organs and tissues. The carboxyhemoglobin complex is about 200 times more stable than the oxygen-hemoglobin complex, leading to symptoms like headaches, weakness, and asphyxia. Soil microorganisms can help reduce carbon monoxide levels.
Hydrocarbons: Consisting only of carbon and hydrogen, hydrocarbons are produced from incomplete combustion of fuels and anaerobic decomposition of organic matter. Methane (CH₄) is a major hydrocarbon pollutant. High levels of hydrocarbons can be carcinogenic and cause plant damage. They also react with nitrogen oxides to create secondary pollutants.
Prevention and Control of Air Pollution
- Combustion: Organic pollutants are burned to convert them into less harmful carbon dioxide (CO₂) and water (H₂O).
- Absorbers: Gaseous pollutants are removed by passing them through absorbers.
- Fabric Filters: Gases containing dust are filtered through fabric to trap particles, which are collected while the purified gases are released.
- Electrostatic Precipitators: Aerosol-containing gases pass through electrostatic precipitators, where particles are electrically charged and collected on electrodes, allowing clean gases to be discharged.
Consequences of Atmospheric Pollution
- Greenhouse Effect: The Earth's atmosphere allows most sunlight to reach and warm the surface. However, greenhouse gases like carbon dioxide, methane, and water vapor trap the heat radiated back from the Earth. This process, known as the greenhouse effect, is crucial for maintaining temperatures that support life on our planet. Without it, Earth would be extremely cold.
- Global Warming: An increase in the concentration of greenhouse gases intensifies the greenhouse effect, leading to global warming. This phenomenon can cause the melting of ice caps and glaciers, resulting in rising sea levels.
Acid Rain
- Formation: Acid rain occurs when oxides of nitrogen and sulfur in the air dissolve in rainwater, forming nitric and sulfuric acids.
- Effects: Acid rain corrodes buildings, monuments (like the Taj Mahal), and statues. It also makes soil more acidic, degrading soil quality and reducing forest and agricultural productivity. Additionally, acid rain leaches heavy metals such as lead, copper, mercury, and aluminum from soil and rocks, contaminating water sources and posing health risks.
- Health Impacts: Lead, often released from vehicle exhausts, is highly toxic and can cause anemia, brain damage, convulsions, and death. Other heavy metals and certain pesticides, like DDT, can lead to kidney and liver damage, and neurological issues.
The Taj Mahal and Marble Cancer
- Issue: The Taj Mahal, a renowned tourist attraction in Agra, faces discoloration of its white marble due to air pollution. Industries around Agra release sulfur dioxide and nitrogen dioxide, which contribute to acid rain. This acid rain accelerates the deterioration of the marble, a phenomenon known as "marble cancer."
- Actions: To protect the Taj Mahal, the Supreme Court has mandated that local industries use cleaner fuels like CNG and LPG, and that vehicles in the area switch to unleaded petrol.
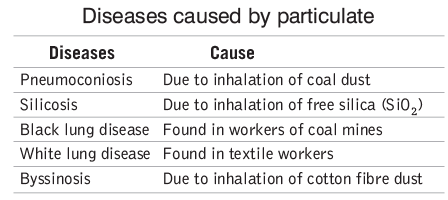
Particulates
- Definition: Particulates are tiny solid particles and liquid droplets suspended in the air, including dust, smoke, mists, and bacteria.
Smog
Classical Smog: This type occurs in cool, humid climates and is primarily composed of sulfur dioxide (SO₂) and particulate matter from burning fuels. It is chemically reducing.
Photochemical Smog: Found in warm, dry, and sunny climates, photochemical smog results from the interaction of primary pollutants like nitrogen oxides and carbon monoxide with secondary pollutants such as ozone and formaldehyde. It is more common in cities with high populations and vehicular traffic and is characterized by its oxidizing nature.- Components and Effects: Ozone in photochemical smog has a pungent smell and can cause respiratory issues and damage rubber products. Peroxyacetyl nitrate (PAN) and aldehydes present in this smog irritate the eyes and are highly toxic to plants, causing damage like bronzing and glazing of leaves.
- Control Measures: Photochemical smog can be mitigated using free radical traps, which react with the precursors of smog. Installing efficient catalytic converters in vehicles also helps reduce the release of nitrogen oxides and hydrocarbons.
Stratospheric Pollution
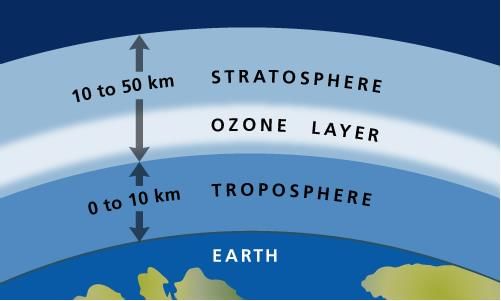
- Ozone Layer and Its Importance: The ozone layer in the stratosphere absorbs harmful ultraviolet (UV) radiation from the Sun, protecting living organisms on Earth.
- Ozone Depletion: When the ozone layer is depleted, it can lead to skin cancer, cataracts, and a reduction in plankton populations in oceans, as well as harm to plant life.
- Causes: The primary cause of ozone depletion is chlorofluorocarbons (CFCs), which are used in refrigeration, fire extinguishers, aerosol sprays, plastic foam production, tubeless tires, and electronic cleaning. These substances persist in the atmosphere for many years. UV rays break down CFCs, releasing chlorine atoms that destroy ozone molecules. One chlorine free radical can destroy up to 1,000 ozone molecules.
- Ozone Hole: Significant depletion, known as the ozone hole, has been observed mainly over Antarctica. This occurs due to the buildup of Polar Stratospheric Clouds (PSCs) and the accumulation of CFCs. The ozone layer tends to recover after the Antarctic spring.
Water Pollution
- Definition: Water pollution is caused by foreign substances, such as sewage, algae, and soluble salts, which contaminate water bodies.
- Contaminants: In some parts of India, drinking water is tainted with arsenic, fluoride, uranium, and other impurities. Major water pollutants include pathogens (like bacteria), organic wastes (such as leaves and grass), and chemical pollutants (including metals from industrial waste and petroleum).
- Dissolved Oxygen (DO): Healthy aquatic life requires a DO level of 5-6 ppm. Levels below 5 ppm can inhibit fish growth.
- Biochemical Oxygen Demand (BOD): This measures the amount of oxygen needed by microorganisms to decompose organic matter in a liter of water.
- Chemical Oxygen Demand (COD): This measures the total amount of oxygen consumed by pollutants in a water sample.
Soil Pollution
- Definition: Soil pollution refers to changes in soil composition caused by substances that reduce soil productivity. Positive soil pollution occurs when productivity declines due to excessive use of fertilizers, pesticides, or air pollutants, while negative soil pollution arises from soil erosion or overuse.
- Landscape Pollution: This occurs when fertile land is converted into barren land due to waste dumping.
- Sources: Main sources of soil pollution include pesticides (e.g., DDT, BHC), herbicides (e.g., sodium chlorate, sodium arsenite), fungicides (e.g., organomercury compounds), and fertilizers. Soil conditioners and various types of solid waste, such as industrial, agricultural, and radioactive waste, also contribute to soil pollution.
Green Chemistry
Overview: Green chemistry focuses on processes and products that minimize or eliminate hazardous substances.
Applications: Examples include:
- Using carbon dioxide as a blowing agent in polystyrene foam production, which eliminates the need for ozone-depleting CFCs.
- Developing ‘sea-nine,’ a safer marine antifouling compound that degrades more rapidly than traditional organotins, which persist and pollute marine environments.
Pollution Control Strategies
- Recycling: This process transforms waste into useful products, such as using scrap metal in steel manufacturing or recovering energy from combusted waste.
- Sewage Treatment: It is essential to control or treat sewage sludge before discharging it into water bodies.
- Incineration: This method converts organic materials into carbon dioxide and water, safely destroying household, chemical, and biological waste. Incineration at high temperatures and with ample oxygen reduces waste volume.
- Biogas Production: Digesting biodegradable waste can produce biogas and manure, useful for treating sewage sludge.
- Oil Zapping: This bioremediation technique uses bacteria to clean oil spills. For instance, 'Oil Zapping' bacteria were employed to address the Mumbai oil spill in August 2010.
Electronic Waste
- Definition: Electronic waste (e-waste) includes discarded computers, office equipment, entertainment devices, mobile phones, televisions, and refrigerators.
- Toxic Components: Computer parts often contain hazardous substances like dioxins, polychlorinated biphenyls (PCBs), cadmium, chromium, radioactive isotopes, and mercury. For example, a typical monitor may contain over 6% lead, mostly in the lead glass of Cathode Ray Tubes (CRTs).
|
468 videos|1404 docs|395 tests
|
FAQs on Pollution and it's Types - General Awareness for SSC CGL
| 1. What are the major gaseous air pollutants that contribute to atmospheric pollution? |  |
| 2. What are the consequences of atmospheric pollution? |  |
| 3. How can stratospheric pollution impact the environment? |  |
| 4. What are some pollution control strategies that can be implemented to reduce atmospheric pollution? |  |
| 5. What are the different types of pollution mentioned in the article and how do they differ? |  |















