Viscosity | General Awareness for SSC CGL PDF Download
Viscosity: Understanding Fluid Resistance and Its Measures
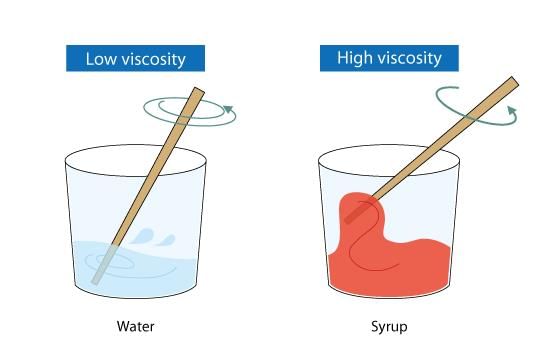 Viscosity, such as in honey, results in a slower flow rate compared to a less viscous fluid like water. The term "viscosity" is derived from the Latin word for mistletoe, viscum, as mistletoe berries produce a viscous glue also called viscum. Common symbols for viscosity include the Greek letters mu (μ) and eta (η). The inverse of viscosity is fluidity.
Viscosity, such as in honey, results in a slower flow rate compared to a less viscous fluid like water. The term "viscosity" is derived from the Latin word for mistletoe, viscum, as mistletoe berries produce a viscous glue also called viscum. Common symbols for viscosity include the Greek letters mu (μ) and eta (η). The inverse of viscosity is fluidity.
Viscosity measures a fluid’s resistance to flow:
- Liquid viscosity decreases with an increase in temperature.
- Gas viscosity increases with an increase in temperature.
Viscosity Units
The SI unit for viscosity is newton-second per square meter (N·s/m²). It is also commonly expressed as pascal-second (Pa·s), kilogram per meter per second (kg·m⁻¹·s⁻¹), poise (P or g·cm⁻¹·s⁻¹ = 0.1 Pa·s), or centipoise (cP), with water at 20 °C having a viscosity of about 1 cP or 1 mPa·s.
In American and British engineering, viscosity may also be measured in pound-seconds per square foot (lb·s/ft²) or pound-force-seconds per square foot (lbf·s/ft²).
How Viscosity Works
- Viscosity is the friction between fluid molecules. Just like when solids rub together, higher viscosity means it's harder to get a fluid to move.
- When you pour a liquid out of a container, there's friction between the liquid's molecules and the container's walls. The molecules near the surface stick more to the container, while those farther away move more freely, only slowed down by bumping into other molecules. Viscosity looks at how fast or slow these molecules flow.
- Several things can change viscosity, like temperature, pressure, and adding other molecules. Pressure doesn't do much to liquids and is usually ignored, but adding more molecules can really change how sticky a liquid is. For instance, if you put sugar in water, it gets thicker.
- Temperature has the biggest effect on viscosity. In liquids, when you heat them up, they get less sticky because the heat gives the molecules the push they need to slide past each other. But in gases, when you raise the temperature, they get stickier because the molecules crash into each other more often, and how much they attract each other matters less in gas viscosity.
Dynamic Viscosity vs. Kinematic Viscosity
Viscosity can be reported in two ways: absolute or dynamic viscosity, which measures a fluid’s resistance to flow, and kinematic viscosity, which is the ratio of dynamic viscosity to a fluid’s density. Two fluids with identical dynamic viscosity values may have different densities, resulting in different kinematic viscosity values. Consequently, dynamic and kinematic viscosity are measured in different units.
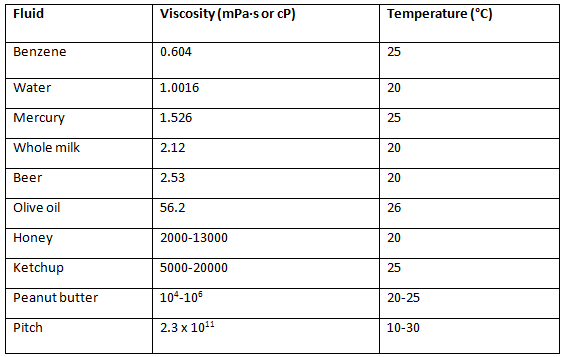
Viscosity Values
Viscosity of Water
 At 20 °C, the dynamic viscosity of water is 1.0016 millipascal-seconds (mPa·s) or 1.0 centipoise (cP). Its kinematic viscosity is 1.0023 centistokes (cSt), 1.0023×10⁻⁶ m²/s, or 1.0789×10⁻⁵ ft²/s. The viscosity of liquid water decreases significantly as temperature increases, with water’s viscosity at 80 °C being 0.354 mPa·s. Conversely, water vapor viscosity increases with rising temperature.
At 20 °C, the dynamic viscosity of water is 1.0016 millipascal-seconds (mPa·s) or 1.0 centipoise (cP). Its kinematic viscosity is 1.0023 centistokes (cSt), 1.0023×10⁻⁶ m²/s, or 1.0789×10⁻⁵ ft²/s. The viscosity of liquid water decreases significantly as temperature increases, with water’s viscosity at 80 °C being 0.354 mPa·s. Conversely, water vapor viscosity increases with rising temperature.
Water has a relatively low viscosity, higher than most liquids with molecules of similar size due to hydrogen bonding between water molecules.
Newtonian and Non-Newtonian Fluids
Newton’s law of friction is crucial for understanding viscosity:
τ = μ dc / dy = μ γ
where:
- τ = shearing stress in the fluid (N/m²)
- μ = dynamic viscosity of the fluid (N·s/m²)
- dc = unit velocity (m/s)
- dy = unit distance between layers (m)
- γ = dy/dc = shear rate (s⁻¹)
Rearranging the terms provides the formula for dynamic viscosity:
μ = τ dy / dc = τ / γ
A Newtonian fluid adheres to Newton’s law of friction, meaning its viscosity is independent of the strain rate. Non-Newtonian fluids do not follow this law and can deviate in various ways:
- Shear-thinning fluids: Viscosity decreases as the shear strain rate increases (e.g., ketchup).
- Shear-thickening fluids: Viscosity increases as the shear strain rate increases (e.g., a suspension of silica particles in polyethylene glycol).
- Thixotropic fluids: Shaking or stirring reduces viscosity (e.g., yogurt).
- Rheopectic or dilatant fluids: Shaking or stirring increases viscosity (e.g., a mixture of cornstarch and water, known as oobleck).
- Bingham plastics: Act as solids under normal conditions but flow as viscous liquids under high stress (e.g., mayonnaise).
Measuring Viscosity
Viscosity is measured using viscometers and rheometers. A rheometer is a specialized type of viscometer. These devices measure either the flow of a fluid past a stationary object or the movement of an object through a fluid, with the viscosity value representing the drag between the fluid and the object’s surface. Accurate measurements require laminar flow and a small Reynolds number.
|
468 videos|1404 docs|395 tests
|
FAQs on Viscosity - General Awareness for SSC CGL
| 1. What is viscosity and why is it important in fluid mechanics? |  |
| 2. What are the different units of viscosity used in scientific measurements? |  |
| 3. How do dynamic viscosity and kinematic viscosity differ? |  |
| 4. What is the viscosity of water at room temperature? |  |
| 5. What are the differences between Newtonian and Non-Newtonian fluids? |  |
















