Case Study Based Questions: Chemical Effects of Electric Current | Science Class 8 PDF Download
Case Study 1
Neha connects two nails to the two terminals of a battery including a torch bulb and a switch in the circuit. She immersed the connection into a solution labelled A in a beaker. When she pass the electric current through the solution A by closing the switch, the bulb starts glowing. Now, she replace the solution A, in the beaker with a solution labelled B. When she switch on the electric current, the bulb does not glow.
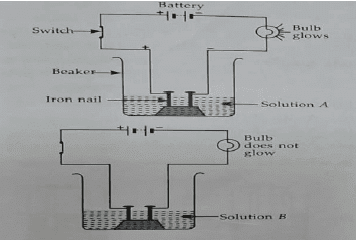
Q1: The solution labelled A would be
(a) Alcohol solution
(b) Hydrochloric acid
(c) Distilled water
(d) None of these
Ans: (b) Hydrochloric acid
Sol: Hydrochloric acid is a conducting solution due to the presence of ions. When Neha passed the electric current through it, the bulb glowed indicating that the solution conducts electricity.
Q2: The solution labelled B would be
(a) Sugar solution
(b) Sulphuric acid solution
(c) Sodium hydroxide
(d) None of these
Ans: (a) Sugar solution
Sol: Sugar solution does not conduct electricity because it does not dissociate into ions in solution. Thus, the bulb did not glow when Neha replaced the solution with sugar solution.
Q3. Which of the following can be used to detect weak current flowing through the liquids in place of the torch bulb?
(a) LED (Light emitting diode)
(b) Compass
(c) Both (a) and (b)
(d) None of these
Ans: (a) LED (Light emitting diode)
Sol: An LED can be used to detect weak currents because it is sensitive to small amounts of current. A compass, on the other hand, is not used for detecting current directly.
Q4: When electric current is passed through a conducting solution, there is a change of colour of the solution. This indicates
(a) Heating effect of electric current
(b) Chemical effect of electric current
(c) Magnetic effect of electric current
(d) Lightning effect of electric current
Ans: (b) Chemical effect of electric current
Sol: The change in color of the solution indicates a chemical reaction occurring due to the electric current, which is the chemical effect of electric current.
Case Study 2
An electric current is passed through acidified water. Bubbles of oxygen gas and hydrogen gas are produced at the two electrodes immersed in it. Oxygen is formed at the positive electrode (anode) which is connected to the positive terminal of the battery and Hydrogen gas is formed at the negative electrode (cathode) which is connected to the negative terminal of the battery. The decomposition of acidified water into hydrogen and oxygen by passing an electric current is an example of electrolysis. Thus, electrolysis is the passage of electricity through a liquid or a solution accompanied by a chemical change.
Q1: Which of the following energy is used to decompose water into its elements?
(a) Heat energy
(b) Light energy
(c) Chemical energy
(d) Electrical energy
Ans: (d) Electrical energy
Sol: Electrolysis of water involves passing an electric current through the water to decompose it into hydrogen and oxygen gases. Thus, electrical energy is used in this process.
Q2: In the process of electrolysis, the current is carried out inside the electrolyte by
(a) Electron
(b) Atoms
(c) Positive and negative ions
(d) All of the above
Ans: (c) Positive and negative ions
Sol: During electrolysis, the electric current is carried through the electrolyte by the movement of positive and negative ions. Electrons move through the external circuit but do not carry the current inside the electrolyte.
Q3: Not pure water but acidic water is used for carrying out electrolysis because
(a) Pure water is a good conductor of electricity
(b) Pure water is a bad conductor of electricity
(c) Pure water is hardly available
(d) None of these
Ans: (b) Pure water is a bad conductor of electricity
Sol: Pure water does not conduct electricity well because it lacks sufficient ions. Acidic water, which contains dissolved acids, increases the conductivity of the water and allows the electric current to pass through more effectively.
Q4: Which of the following is the application of Electrolysis?
(a) Extraction of metals
(b) Purification of metals
(c) Electroplating
(d) All of these
Ans: (d) All of these
Sol: Electrolysis has several applications, including the extraction of metals from ores, purification of metals, and electroplating to coat surfaces with a layer of metal.
|
92 videos|296 docs|44 tests
|
















