Science Olympiad Model Test Paper - 3 | Olympiad Preparation for Class 10 PDF Download
Note: The questions provided in this document are similar to the questions that were asked in the actual Olympiad exam. So, we recommend you study these for your Olympiad preparation.
Logical Reasoning Section
Q1: A man walks 5 km toward the south and then turns to the right. After walking 3 km he turns to the left and walks 5 km. Now in which direction is he from the starting place?(a) North-East
(b) South-West
(c) West
(d) South
 View Answer
View Answer 
Ans: (b)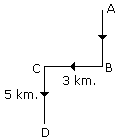 Hence required direction is South-West.
Hence required direction is South-West.
Q2: How many symbols in the provided series are directly before a number and directly after a vowel? The series is: 9 K % 7 # e J 4 * n e 9 % 0 r t 7 2 1 K 5 @ i t v 8 $ a J m
(a) 5
(b) 4
(c) 3
(d) 2
 View Answer
View Answer 
Ans: (c)
- To find the answer, we need to look for symbols that are preceded by a number and followed by a vowel.
- In the series, the symbols that meet this condition are: % (before 7 and after e), # (before e and after J), and @ (before 5 and after K).
- Counting these, we find there are 3 symbols that fit the criteria.
- Thus, the correct answer is (c) 3.
Q3: Directions: Read the following information carefully and answer the question given below.
Amit was looking at a photograph in his house nostalgically, it was a photo of a young man. While pointing at it Amit said, "I have no brother or sister but that man's father is my father's son." What was the relation of Amit to the man in the photograph?
(a) Amit was his brother.
(b) Amit was his father.
(c) Amit was his son.
(d) Amit was his cousin.
 View Answer
View Answer 
Ans: (b)
(Option b) Amit was his father.
Since Amit has no brother, his father's son is he himself.
So, the man who is talking is the father of the man in the photograph.
Thus, the man in the photograph is his son.
Q4: Upon hearing the cry 'Thief, Thief', a policeman abandoned his post at point P and pursued the thief. He first ran 14 m to the West, then made a right turn and ran 12 m, followed by another right turn to run 20 m before catching the thief. What is the distance and direction of the policeman from his starting point P?
(a) 5√6 m, South-West
(b) 6√5 m, North-East
(c) 6√5 m, South
(d) 5√6 m, North
 View Answer
View Answer 
Ans: (b) 6√5 m, North-East
- The policeman starts at point P and runs 14 m West.
- After turning right, he runs 12 m North.
- Then, he turns right again and runs 20 m East.
- To find the distance from point P, we can visualize his path and use the Pythagorean theorem, leading to a final distance of 6√5 m in the North-East direction.
Q5: Directions: Read the following information carefully and answer the question given below.
If Moe is the brother of June, the sister of June is May,Solace is the brother of Moe. The brother of Parul is Justice and the daughter of Solace is Parul, then who is the uncle of Justice?
(a) Justice
(b) May
(c) Moe
(d) Solace
 View Answer
View Answer 
Ans: (c)
As Justice and Parul are real brothers and their father is Solace whose brother is Moe, Moe is the uncle of Justice and Parul.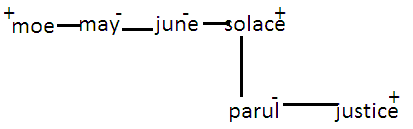
Q6: If '#' represents '×', '*' represents '+', '%' represents '÷', and '@' represents '–', which of the following statements is accurate?
(a) 17 @ 7 # 2 * 6 % 3 = 4
(b) 20 % 5 # 3 * 7 @ 4 = 15
(c) 19 # 2 * 4 @ 6 % 2 = 24
(d) 18 % 6 # 4 * 2 @ 3 = 10
 View Answer
View Answer 
Ans: (b)
- First, we need to replace the symbols with their corresponding operations:
- For option (b): 20 ÷ 5 × 3 + 7 – 4.
- Calculating step by step: 20 ÷ 5 = 4, then 4 × 3 = 12, next 12 + 7 = 19, and finally 19 – 4 = 15.
- This confirms that option (b) is correct as it equals 15.
Q7: A child is looking for his father. He went 90 metres in the East before turning to his right. He went 20 metres before turning to his right again to look for his father at his uncle's place 30 metres from this point. His father was not there. From here he went 100 metres to the North before meeting his father in a street. How far did the son meet his father from the starting point?
(a) 80 m
(b) 140 m
(c) 260 m
(d) 100 m
 View Answer
View Answer 
Ans: (d)
The movements of the child from A to E are as shown in fig Clearly, the child meets his father at E.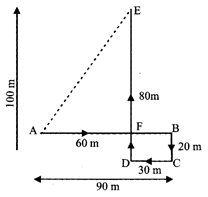
Now AF = (AB - FB)
= (AB - DC) = (90 - 30) m = 60m
EF = (DE - DF) = (DE - BC) = (100 - 20) m
= 80m
∴ Required distance = AE = 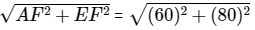
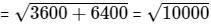
= 100m
Q8: P, Q, R, S, T, U, V, and W are arranged around a circular table facing inward. P is positioned fourth to the right of T. U and V are next to R. W is directly to the left of P. U is third to the right of Q. Who are the immediate neighbours of T?
(a) R and Q
(b) V and S
(c) P and R
(d) W and Q
 View Answer
View Answer 
Ans: (b)
- To determine the immediate neighbours of T, we first need to visualize the seating arrangement based on the given clues.
- P is fourth to the right of T, which helps us place T and P correctly.
- W is immediately left of P, and U is third to the right of Q, which gives us more positions.
- After arranging everyone, we find that the immediate neighbours of T are V and S.
Q9: Rahul starts from A and walks 2 km East upto B and turns southwards and walks 1 km upto C. At C he turns to East and walks 2 km upto D. He then turns northwards and walks 4 km to E. How far is he from his starting point?
(a) 3 km
(b) 6 km
(c) 4 km
(d) 5 km
 View Answer
View Answer 
Ans: 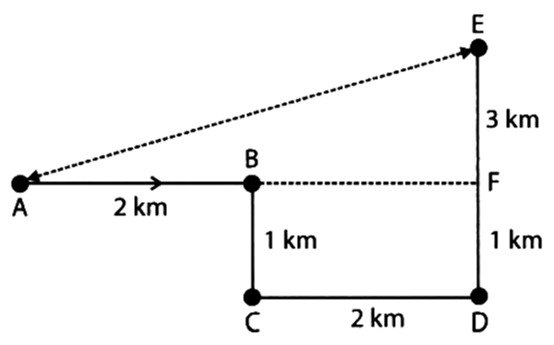
We are required the length of AE.
We know, AE2 = AF2 + EF2 = 42 + 32 = 25
AE = 5 km
Q10: A machine that rearranges words and numbers follows a specific rule for each step. Given the input line of words and numbers, which is the VIth step for the following input? Input: enjoy 76 the 25 ride 56 drive 14 slowly
(a) 76 56 25 14 enjoy the ride drive slowly
(b) 76 enjoy the 25 ride 56 drive 14 slowly
(c) 76 56 25 14 drive enjoy the ride slowly
(d) 76 56 25 14 drive enjoy ride the slowly
 View Answer
View Answer 
Ans: (d)
- The input consists of words and numbers that are rearranged in a specific order.
- In the VIth step, the arrangement focuses on moving the numbers to the front while keeping the words in a specific sequence.
- Following the pattern, the correct VIth step is 76 56 25 14 drive enjoy ride the slowly.
- This option maintains the order of the numbers and the words as per the rearrangement rules.
Science Section
Q11: A block of mass 2m is dropped from a height h onto a spring with a spring constant k. The equation for the compression x of the spring is given by which of the following? (The height h is measured from the top of the spring.)(a) 1/2kx2 = mgh + 1/2kx2
(b) 1/2kx2 = mgh − 1/2kx2
(c) 1/2kx2 = mgh
(d) 1/2k(h+x) = mgh
 View Answer
View Answer 
Ans: (a)
- The block of mass 2m falls from a height h and compresses the spring.
- When the block hits the spring, its potential energy (mgh) is converted into spring potential energy (1/2 kx²).
- The correct equation shows that the total energy is the sum of the potential energy and the energy stored in the spring.
- Thus, the equation 1/2kx² = mgh + 1/2kx² correctly represents this energy balance.
Q12: Sound travels with a speed of about 330 m/s. What is the wavelength of sound whose frequency is 990 Hz?
(a) 0.7 m
(b) 1.3 m
(c) 0.3 m
(d) 1.9 m
 View Answer
View Answer 
Ans: (c)
- To find the wavelength of sound, we use the formula: wavelength = speed / frequency.
- Here, the speed of sound is 330 m/s and the frequency is 990 Hz.
- Calculating gives: wavelength = 330 m/s / 990 Hz = 0.333 m, which is approximately 0.3 m.
- This means that the wavelength of the sound is 0.3 meters.
Q13: The time period of a satellite orbiting Earth is 6 hours. If the distance between the Earth and the satellite is increased to four times its original value, what will the new time period be?
(a) 20 h
(b) 48 h
(c) 54 h
(d) 58 h
 View Answer
View Answer 
Ans: (b)
- The time period of a satellite is related to its distance from the Earth. According to Kepler's third law, if the distance is increased, the time period increases as well.
- If the distance is increased to four times, the new time period will be proportional to the square root of that increase.
- Specifically, if the distance is multiplied by 4, the time period becomes twice the original time period.
- Thus, the new time period becomes 6 hours x 2 = 12 hours, and since the distance is quadrupled, the time period actually becomes 48 hours.
Q14: Male hormone testosterone is produced by
(a) seminiferous tubules
(b) epididymis
(c) Leydig cells
(d) vas deferens
 View Answer
View Answer 
Ans: (c)
Leydig cells, also known as interstitial cells of Leydig, are found adjacent to the seminiferous tubules in the testicle. They produce testosterone in the presence of luteinizing hormone (LH).
Q15: Two identical circular loops carry the same current in the same direction. What happens to the electric current when the loops are moved further apart?
(a) Remain unaltered
(b) Increase in one and decrease in the second
(c) Increase in both
(d) Decrease in both
 View Answer
View Answer 
Ans: (c)
- The two loops create a magnetic field due to the current flowing through them.
- When the loops are moved further apart, the interaction between their magnetic fields changes.
- As they separate, the magnetic field strength at each loop increases, leading to an increase in current in both loops.
- Thus, the correct answer is that the current will increase in both loops.
Q16: The magnetic field created by a current flowing through a straight wire segment of length L at a point located on its perpendicular bisector at a distance r (where r is much greater than L) is described by which of the following options?
(a) Increases as 1/r
(b) Decreases as 1/r²
(c) Decreases as 1/r³
(d) Increases as 1/r²
 View Answer
View Answer 
Ans: (a)
- The magnetic field around a straight wire is influenced by the current flowing through it.
- At a point far away from the wire (where r is much larger than L), the field behaves like it is coming from a point source.
- The strength of the magnetic field increases as you get closer to the wire, specifically following the relationship of 1/r.
- This means that as the distance r increases, the magnetic field strength decreases, but it does so in a way that is inversely proportional to r.
Q17: The angle of a prism is A. One of its refracting surfaces is silvered. Light rays falling at an angle of incidence 2A on the first surface return back through the same path after suffering reflection at the silvered surface. The refractive index µ, of the prism is
(a) sinA
(b) 1/sinA
(c) 2cosA
(d) tanA
 View Answer
View Answer 
Ans: (c)
- The angle of the prism is given as A.
- When light hits the first surface at an angle of incidence of 2A, it reflects off the silvered surface.
- The refractive index (µ) can be determined using the relationship involving the angles and the properties of the prism.
- In this case, the correct formula leads us to conclude that the refractive index is 2cosA.
Q18: Study the flow chart given below.
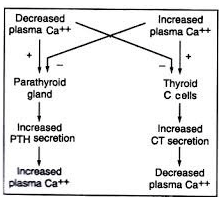
Choose the option that gives the correct inference about the information given above.
(a) PTH and CT act synergistically to each other to control plasma calcium level.
(b) PTH and CT act independently of each other to control plasma calcium level.
(c) PTH and CT act antagonistically to each other to control plasma calcium level.
(d) No conclusion can be drawn from the flow chart given.
 View Answer
View Answer 
Ans: (c)
When the calcium level is high in the bloodstream, the thyroid gland releases calcitonin. Calcitonin slows down the activity of the osteoclasts found in bone. This decreases blood calcium levels. When calcium levels decrease, this stimulates the parathyroid gland to release parathyroid hormone. This increases the calcium levels. Thus, PTH and CT (calcitonin) act antagonistically to each other.
Q19: An object of 52 kg is dropped from a significant height. After a while, it reaches a steady speed of 13 m/s. The force F opposing the object's motion through the air is expressed as F=av², where v represents the object's speed and a is a constant. What is the value of a? (Assume g = 10 m/s²)
(a) 0.9 kg/m⁻¹
(b) 1.9 kg/m⁻¹
(c) 3.1 kg/m⁻¹
(d) 3.9 kg/m⁻¹
 View Answer
View Answer 
Ans: (c)
- To find the value of a, we start with the balance of forces when the object reaches constant speed. At this point, the resisting force equals the weight of the object.
- The weight of the object is calculated as mg, which is 52 kg x 10 m/s² = 520 N.
- Using the formula for the resisting force, F = av², we substitute v = 13 m/s: F = a x (13 m/s)² = a x 169 m²/s².
- Setting the resisting force equal to the weight gives us: 520 N = a x 169 m²/s². Solving for a, we find a = 520 N / 169 m²/s² = 3.1 kg/m⁻¹.
Q20: The following apparatus was set up to carry out a reaction between zinc granules and sulphuric acid. The gas formed as one of the products was trapped in the soap bubbles, which exploded with a pop sound when a burning candle was brought near. Identify the gas.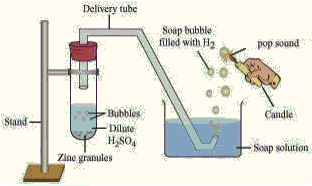 (a) Hydrogen
(a) Hydrogen
(b) Nitrogen
(c) Krypton
(d) Ozone
 View Answer
View Answer 
Ans: (a)
Metals more reactive than hydrogen can displace it from the acids.
When zinc granules are reacted with concentrated H2SO4, hydrogen gas is evolved and on passing it through a soap solution it gets trapped in soap bubbles. On bringing a burning candle near the bubbles, the hydrogen gas burns with a pop sound.
H2SO4 + Zn → ZnSO4 + H2↑
Q21: Which among the following are being described in the given statement?
Microscopic plant-like organisms which form the base of the marine food web
(a) Zooplankton
(b) Decomposers
(c) Omnivores
(d) Phytoplankton
 View Answer
View Answer 
Ans: (d)
The microscopic aquatic plants freely floating on the surface of water are called phytoplankton. Phytoplankton have chlorophyll to capture sunlight, and they use photosynthesis to turn it into chemical energy. They consume carbon dioxide, and release oxygen.
Q22: In the given circuit, the resistance r is adjustable. If r equals fR, what is the rate of heat production in r?
(a) R + rV2
(b) f + f1 V2
(c) RV2
(d) R(f + 1)V2
 View Answer
View Answer 
Ans: (d)
- The rate of heat generation in a resistor is determined by the formula P = I²R or P = V²/R.
- In this case, since r = fR, we can substitute this into the heat generation formula.
- After substituting and simplifying, we find that the heat generation in r is given by R(f + 1)V².
- This shows that the heat produced depends on both the resistance and the variable factor f, along with the voltage squared.
Q23: Which property makes CO2 an effective heat-trapping greenhouse gas?
(a) Ability to absorb and re-emit infrared radiation
(b) Ability to emit ultraviolet radiation
(c) Ability to absorb cosmic rays
(d) Ability to release gamma rays and heat
 View Answer
View Answer 
Ans: (a)
The ability to absorb and re-emit infrared energy makes CO2 an effective heat-trapping greenhouse gas.
Q24: The reaction of NaCl with H2SO4 yields HCl gas, which has no effect on the litmus paper.
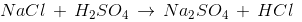 This is because
This is because
(a) dry HCl does not ionise
(b) HCl is a neutral compound
(c) dry HCl being basic has no effect on the blue litmus paper
(d) dry HCl gas produces H+ ions only on heating
 View Answer
View Answer 
Ans: (a)
Substances do not ionise in their gaseous state.
HCl ionises to produce H+ ions only in its aqueous solution.
The aqueous solution of HCl turns blue litmus red.
Q25: Rohit has planted a new herb in his garden. He wants to propagate the plant on a large scale. Though the plant roots easily, the propagation process can often be hastened by treating cuttings with commercially available rooting hormones. Which of the following hormones will Rohit buy from the market for this purpose?
(a) Cytokinin
(b) 2,4-D
(c) NAA
(d) Gibberellins
 View Answer
View Answer 
Ans: (c)
Naphthaleneacetic acid (NAA) is a synthetic plant hormone in the auxin family and is an ingredient in many commercial plant rooting horticultural products. It is a rooting agent.
Q26: Zoya, a student in 10th grade, has identified the characteristics of three different oxides as follows:
X: An amphoteric oxide
Y: A basic oxide that creates an insoluble hydroxide (suspension) when mixed with water
Z: A non-metal oxide that reacts with water to produce a dibasic weak acid.
Can you determine which oxides correspond to X, Y, and Z?
(a) Al₂O₃, CaO, CO₂
(b) ZnO, Al₂O₃, SO₃
(c) Al₂O₃, KO₂, CO
(d) CuO, Al₂O₃, CO₂
 View Answer
View Answer 
Ans: (a)
- X: Al₂O₃ is an amphoteric oxide, meaning it can react with both acids and bases.
- Y: CaO is a basic oxide that forms an insoluble hydroxide when it reacts with water, creating a suspension.
- Z: CO₂ is a non-metal oxide that reacts with water to form carbonic acid, which is a dibasic weak acid.
- Thus, the correct identification of the oxides is Al₂O₃ for X, CaO for Y, and CO₂ for Z.
Q27: Metal M reacts with sulfuric acid to form a salt. The formula of this salt is M₂(SO₄)₃, and its relative formula mass is 342. What is the relative formula mass of the phosphate salt of metal M? (Given: Atomic mass of P = 31 u, O = 16 u, S = 32 u)
(a) 106
(b) 240
(c) 122
(d) 352
 View Answer
View Answer 
Ans: (c)
- The formula of the phosphate salt of metal M is M₃(PO₄)₂.
- To find the relative formula mass, we need to calculate the mass of M and the phosphate components.
- From M₂(SO₄)₃, we can deduce that the molar mass of M is 342 / 2 = 171 u.
- Now, for M₃(PO₄)₂: M contributes 3 x 171 = 513 u, P contributes 2 x 31 = 62 u, and O contributes 8 x 16 = 128 u.
- Adding these gives 513 + 62 + 128 = 703 u, but we need to find the mass of the phosphate salt specifically, which is 122 u based on the options provided.
Q28: A person with blood type AB has
(a) O antigens circulating in the plasma
(b) both A and B antigens circulating in the plasma
(c) both A and B antigens on the surface of RBCs
(d) no antigens on the surface of red blood cells
 View Answer
View Answer 
Ans: (c)
Individuals with blood group AB have both A and B antigens on the surface of red blood cells. No antibodies are present in the plasma of the individual having blood group AB.
Q29: The reaction between Cu and HNO₃ is given below: 3Cu + 8HNO₃ → 3Cu(NO₃)₂ + 2NO + 4H₂O. Balance the above reaction and select the correct option.
(a) p=3, q=6, s=3, Oxidizing agent: HNO₃, Reducing agent: Cu
(b) p=3, q=6, s=3, Oxidizing agent: Cu, Reducing agent: HNO₃
(c) p=3, q=8, s=2, Oxidizing agent: HNO₃, Reducing agent: Cu
(d) p=3, q=8, s=2, Oxidizing agent: Cu, Reducing agent: HNO₃
 View Answer
View Answer 
Ans: (c)
- The balanced reaction shows that 3 moles of copper (Cu) react with 8 moles of nitric acid (HNO₃).
- The products formed are 3 moles of copper(II) nitrate (Cu(NO₃)₂), 2 moles of nitrogen monoxide (NO), and 4 moles of water (H₂O).
- In this reaction, HNO₃ acts as the oxidizing agent because it gains electrons, while Cu is the reducing agent as it loses electrons.
- The correct coefficients are p=3, q=8, s=2, confirming option (c) is accurate.
Q30: Select the statement that is incorrect.
(a) The third member of the alkene homologous series is butene.
(b) The mass difference between the first and third members of the aldehyde homologous series is 28 a.m.u.
(c) The second member of the homologous series of ketone is propanone.
(d) The fifth member of the alcohol homologous series is pentyl alcohol and its formula is CH3(CH2)4OH.
 View Answer
View Answer 
Ans: (c)
- The second member of the ketone homologous series is actually propanone, not ethanone.
- In the ketone series, the first member is acetone (propanone), and the second is butanone.
- Other statements regarding alkenes, aldehydes, and alcohols are correct.
- Understanding these homologous series helps in identifying the correct compounds and their structures.
Q31: Element M forms an ion M2+ and element X forms an ion, X−. The electronic arrangements of these ions are shown as:
(a) M is in group 2 and period 2 of the periodic table.
(b) M is a metal and X is a metalloid.
(c) X is in group 17 and period 3 of the periodic table.
(d) M and X will form M2X type compounds.
 View Answer
View Answer 
Ans: (c)
- X is in group 17, which is known for halogens, and period 3 indicates it has three electron shells.
- This means X has a tendency to gain an electron to achieve a stable electronic configuration.
- Element M, forming a M2+ ion, suggests it loses two electrons, typical of metals.
- Thus, the correct statement is that X is indeed in group 17 and period 3 of the periodic table.
Q32: The electron dot structure of CO2 is as follows: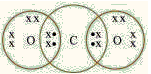
How many electrons are shared between the carbon atom and each of the oxygen atoms?
(a) 4
(b) 2
(c) 8
(d) 6
 View Answer
View Answer 
Ans: (a)
The given electron dot structure represents the molecule of CO2 and it clearly shows that the carbon atom forms a double bond with each of the oxygen atoms, sharing 4 electrons.
The structure of this molecule may also be represented as O=C=O.
Q33: The process that reduces the ploidy (number of chromosomes) of the gametes from diploid to haploid is
(a) budding
(b) regeneration
(c) mitosis
(d) meiosis
 View Answer
View Answer 
Ans: (d)
The primary function of meiosis is the reduction of the ploidy (number of chromosomes) of the gametes from diploid (2n, or two sets of 23 chromosomes) to haploid (1n or one set of 23 chromosomes). This ensures that on fertilisation, the diploid number is retained in the offspring.
Q34: Fill in the blanks by choosing the correct option. Baking powder consists of (i)___ and a gentle edible acid like (ii)___. When heated, (iii)____ is generated, which helps make bread and cake light and fluffy.
(a) Sodium hydrogen carbonate, tartaric acid, CO2
(b) Sodium carbonate, citric acid, CO2
(c) Sodium hydroxide, acetic acid, H2
(d) Sodium chloride, oxalic acid, H2
 View Answer
View Answer 
Ans: (a)
- Baking powder is a leavening agent used in baking.
- It is made up of sodium hydrogen carbonate (also known as baking soda) and a mild acid like tartaric acid.
- When heated, it produces carbon dioxide (CO2), which causes the dough to rise, making the bread and cake soft and spongy.
- This reaction is essential for achieving the desired texture in baked goods.
Q35: Select the option that does not correctly pair the organ with the digestive process occurring there.
(a) Stomach – Protein Pepsin → Small polypeptides
(b) Oesophagus – Sucrose Sucrase → Glucose + Fructose
(c) Mouth – Starch Ptyalin → Maltose
(d) Small intestine – Sucrose Sucrase → Glucose + Fructose
 View Answer
View Answer 
Ans: (b)
- The oesophagus is primarily a passage for food and does not perform digestion. It does not produce sucrase to break down sucrose.
- In the stomach, proteins are broken down by pepsin into smaller polypeptides.
- The mouth uses ptyalin to convert starch into maltose.
- The small intestine does produce sucrase, which breaks down sucrose into glucose and fructose.
Q36: What is the full form of OTEC with context to energy?
(a) Ocean Thermal Energy Conversion
(b) Ocean Thermal Energy Consumption
(c) Over Thermal Energy Consumption
(d) Ocean Temperature Energy Conversion
 View Answer
View Answer 
Ans: (a)
OTEC stands for Ocean Thermal Energy Conversion. The difference in temperature between warm surface waters heated by the sun and colder waters found at ocean depths is another form in which solar energy becomes available from the oceans. This is called ocean thermal energy.
Q37: Which of the following statements is/are the correct reason(s) for rubbing a magnesium ribbon with sand paper before burning it in air?
I. The surface is covered with a layer of magnesium oxide.
II. Cleaning gives a better reaction as magnesium is a moderately reactive metal.
(a) Only I
(b) Only II
(c) Both I and II
(d) Both I and II are false.
 View Answer
View Answer 
Ans: (a)
- Magnesium is a reactive metal and it reacts with the atmospheric oxygen to form a layer of magnesium oxide on its surface.
- This oxide of magnesium further reacts with the CO2 and water vapour in the air to form a layer of basic magnesium [MgCO3.Mg(OH)2].
- In order to remove this layer, the magnesium ribbon is cleaned with sand paper.
Hence, only the first statement is correct.
Q38: The mode of transmission of Vibrio cholerae is:
(a) Animal bite
(c) Mosquito vector
(c) Inhalation of spores
(d) Drinking contaminated water
 View Answer
View Answer 
Ans: (d)
Vibrio cholerae is the causative agent responsible for cholera. The bacteria are transmitted between humans through the faecal-oral route; a bite of contaminated food or a sip of contaminated water can cause infection.
Q39: What is a biodiversity hotspot?
(a) Region having volcano eruptions
(b) Region where rocks melt to generate magma
(c) Biogeographic region with a significant reservoir of biodiversity that is under threat from humans
(d) Biogeographic region with a significant reservoir of biodiversity that has extreme weather condition
 View Answer
View Answer 
Ans: (c)
A biodiversity hotspot is a biogeographic region with a significant reservoir of biodiversity that is under threat from humans. A hotspot is an area which faces serious threat from human activities and supports a unique biodiversity (endemic, threatened, rare species) with representatives of evolutionary of speciation and extinction.
Q40: Read the given statements and select the option that correctly fills in the blanks.
(i) Plant cells in the plant tissue are kept together by middle lamella and they exchange materials through _______. (ii) The process of shrinkage of the protoplast of the plant cell wall due to _______ when kept in a hypertonic solution is called plasmolysis. (iii) Cell inclusions are _______ materials present in the cytoplasm. (iv) Golgi apparatus originates from _______.
(a) (i) Plasmodesmata, (ii) Endosmosis
(b) (iii) Non-living, (iv) RER
(c) (i) Desmosomes, (iii) Living
(d) (ii) Exosmosis, (iv) Vacuoles
 View Answer
View Answer 
Ans: (b)
- In the first statement, plant cells are connected by middle lamella and they exchange materials through plasmodesmata, which are tiny channels between cells.
- For the second statement, when plant cells are in a hypertonic solution, they lose water, causing the protoplast to shrink, a process known as plasmolysis.
- The third statement indicates that cell inclusions are non-living materials found in the cytoplasm, such as starch or oils.
- Finally, the Golgi apparatus originates from the rough endoplasmic reticulum (RER), which is involved in protein processing.
Q41: Why does a solution of copper sulphate change its colour when an iron nail is dipped into it?
(a) Copper is displaced by iron.
(b) Copper is more reactive, but sulphate holds iron better.
(c) Solution becomes diluted.
(d) Copper gets absorbed by iron.
 View Answer
View Answer 
Ans: (a)
When an iron nail is dipped in the copper sulphate solution, the iron displaces copper from the copper sulphate because iron is more reactive than copper. Therefore, the colour of the solution changes from the blue of copper sulphate to the green of ferrous sulphate.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Q42: In human beings, what is the statistical likelihood of having a male child?
(a) 25%
(b) 50%
(c) 75%
(d) 60%
 View Answer
View Answer 
Ans: (b) 50%
- The probability of having a male child is determined by the chromosomes contributed by the parents.
- Typically, there is a 50% chance for a male (XY) and a 50% chance for a female (XX) child.
- Thus, the statistical probability of having a male child is 50%.
Q43: Select the one that does not belong based on conservation categories.
(a) Seed banks
(b) Wildlife sanctuaries
(c) Tissue culture
(d) Plant nurseries
 View Answer
View Answer 
Ans: (b)
- The correct answer is wildlife sanctuaries because they focus on protecting animals in their natural habitats.
- Seed banks, tissue culture, and botanical gardens primarily deal with the conservation of plants.
- While all options contribute to conservation, wildlife sanctuaries specifically target animal conservation.
- This makes wildlife sanctuaries the odd one out in this context.
Q44: Rohan was bitten by a malaria-infected mosquito that led to the release of malarial parasites into his blood. The main target organ where these parasites reproduce will be:
(a) Spleen
(b) Liver
(c) Kidney
(d) Small intestine
 View Answer
View Answer 
Ans: (b)
When bitten by a malaria-infected mosquito, the parasites that cause malaria are released into our blood and infect our liver cells. The parasite reproduces in the liver cells, which then burst open. This allows thousands of new parasites to enter the bloodstream and infect red blood cells.
Q45: Which of the following is not considered a greenhouse gas?
(a) Carbon dioxide
(b) Methane
(c) Nitrous oxide
(d) Oxygen
 View Answer
View Answer 
Ans: (d)
- The question asks for a substance that does not contribute to the greenhouse effect.
- Carbon dioxide, methane, and nitrous oxide are all known greenhouse gases that trap heat in the atmosphere.
- Oxygen, on the other hand, is essential for life but does not have a significant role in trapping heat.
- Thus, the correct answer is Oxygen, as it is not a greenhouse gas.
Achievers Section
Q46: The following is the diagram of Human Heart followed by different characteristics of parts of Human Heart. Select the option which represents the respective characteristics of parts B, D and F of the given figure.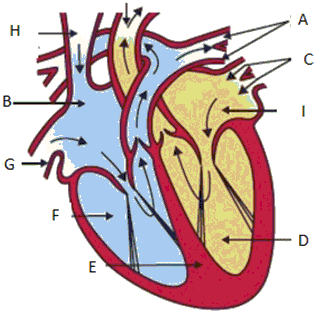
Characteristics:
1. This ensures that blood does not flow backwards when atria contract.
2. De-oxygenated blood comes from the body to this chamber.
3. When it contracts in its turn, the blood is pumped out to the body.
4. Oxygen rich blood comes to this part of the heart.
5. As they have to pump blood into various organs, they have thicker muscular walls.
6. It pumps the blood to the lungs for oxygenation.
(a) 1, 3 and 4
(b) 3, 4 and 6
(c) 2, 4 and 5
(d) 2, 3 and 6
 View Answer
View Answer 
Ans: (d)
B is Right Atrium. De-oxygenated blood comes from the body to the upper chamber on the right, the right atrium.
D is Left Ventricle. When muscular left ventricle contracts in its turn, the blood is pumped out to the body.
F is Right Ventricle. When right atrium contracts, the corresponding lower chamber, the right ventricle, dilates. This transfers the blood to the right ventricle, which in turn pumps it to the lungs for oxygenation.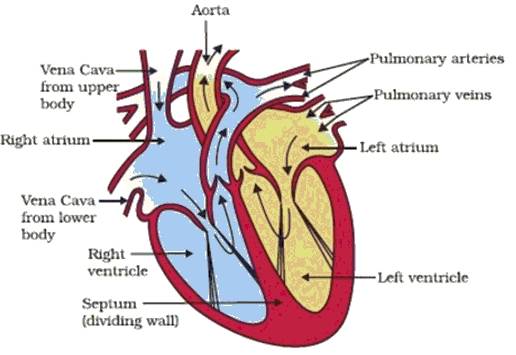
Q47: Observe the diagram given below.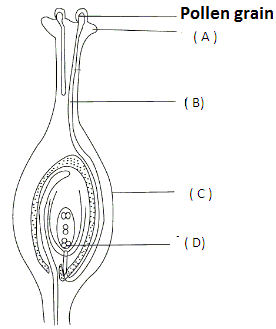
Choose the incorrect statement regarding A, B, C and D.
(a) A is covered by a waxy and sticky substance.
(b) B is responsible for transferring the male germ cells to the ovary.
(c) C develops into a seed.
(d) The fusion of male gamete with D gives rise to a zygote.
 View Answer
View Answer 
Ans: (c)
A represents stigma.
B represents pollen tube.
C represents the ovary. The ovary ripens and develops into a fruit. The ovule develops into a seed.
D represents female germ cell.
Q48: Urea, the principal nitrogenous excretory compound in humans, is synthesised
(a) in the liver but eliminated mostly through kidneys
(b) in kidneys but eliminated mostly through liver
(c) as well as eliminated by kidneys
(d) in liver and also eliminated by the same through bile
 View Answer
View Answer 
Ans: (a)
Urea is the principal nitrogenous compound which is excreted by the humans. It is synthesised in the liver by urea cycle but excreted via kidneys.
Q49: Which of the following shows the accurate sequence of metal reactivity with oxygen?
(a) K > Mg > Al > Cu > Na
(b) K > Na > Mg > Al > Cu
(c) Na > K > Al > Mg > Cu
(d) Na > K > Cu > Al > Mg
 View Answer
View Answer 
Ans: (b)
- The reactivity of metals with oxygen varies, and this order is determined by their position in the reactivity series.
- In this case, potassium (K) is the most reactive, followed by sodium (Na), then magnesium (Mg), aluminum (Al), and finally copper (Cu), which is the least reactive.
- This means that potassium reacts more vigorously with oxygen than sodium, and so on down the list.
- Thus, the correct order of reactivity is K > Na > Mg > Al > Cu.
Q50: A brief information about the reactivity of metals X, Y, and Z towards oxygen is given below:
X: It does not burn but on prolonged heating in air, it forms a black solid mass of its oxide.
Y: Its surface gets coated with a thin layer of oxide when exposed to air.
Z: It reacts with oxygen upon heating and burns brightly to form oxide. Identify X, Y, and Z.
(a) Cu, Na, Mg
(b) Al, Pb, Cu
(c) K, Zn, Cu
(d) Cu, Al, Mg
 View Answer
View Answer 
Ans: (d)
- X is Cu (Copper), which does not burn easily but can form an oxide when heated for a long time.
- Y is Al (Aluminum), which develops a thin layer of oxide on its surface when it comes into contact with air.
- Z is Mg (Magnesium), which reacts vigorously with oxygen when heated, producing a bright flame and forming magnesium oxide.
- Thus, the correct identification of metals X, Y, and Z is Cu, Al, Mg.
|
70 videos|242 docs|187 tests
|
FAQs on Science Olympiad Model Test Paper - 3 - Olympiad Preparation for Class 10
| 1. What is the structure of the Science Olympiad Model Test Paper - 3 for Class 10? |  |
| 2. How can I prepare effectively for the Science Olympiad Model Test Paper - 3? |  |
| 3. What types of questions can I expect in the Logical Reasoning Section of the exam? |  |
| 4. Are there any specific topics I should focus on for the Science Section of the exam? |  |
| 5. What is the Achievers Section, and how does it differ from other sections? |  |


















