Mnemonics: Redox Reaction | Chemistry Class 11 - NEET PDF Download
Redox Reactions
Get ready to simplify your study of Redox Reactions! These mnemonics will make understanding oxidation, reduction, and balancing redox reactions both fun and easy to remember.
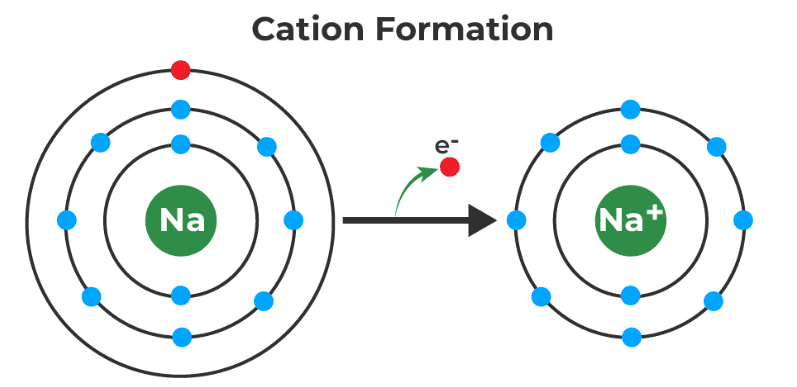
Cation
Describes: Cations are positively (+) charged ions.
- Mnemonics: "Cats Have Paws "
- Cats: Cations (positively charged ions).
- Paws: A playful way to remember that cations are positive.
Anion
Describes: Anion are negatively (-) charged ions.
- Mnemonics: "An ion ⇒ Anion."
- An: A Negative
- Ion: Ion

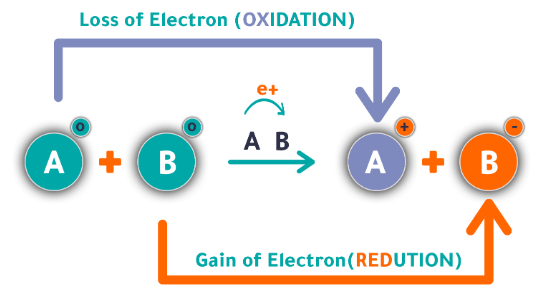
Oxidation and Reduction
Describes: A redox reaction involves the transfer of electrons between reactants. One substance gets oxidized (loses electrons) and another gets reduced (gains electrons).
- Mnemonic: "LEO the lion says GER."
- LEO: Loss of Electrons is Oxidation
- GER: Gain of Electrons is Reduction
Oxidizing Agent and Reducing Agent
Describes: The substance that causes oxidation by accepting electrons is called the oxidizing agent. The substance that causes reduction by donating electrons is called the reducing agent.
- Mnemonic: "Reduce with the Donor, Oxidize with the Receiver"
- Reduce with the Donor: The reducing agent is the one donating electrons to reduce the other substance.
- Oxidize with the Receiver: The oxidizing agent receives electrons and causes the oxidation of the other substance.

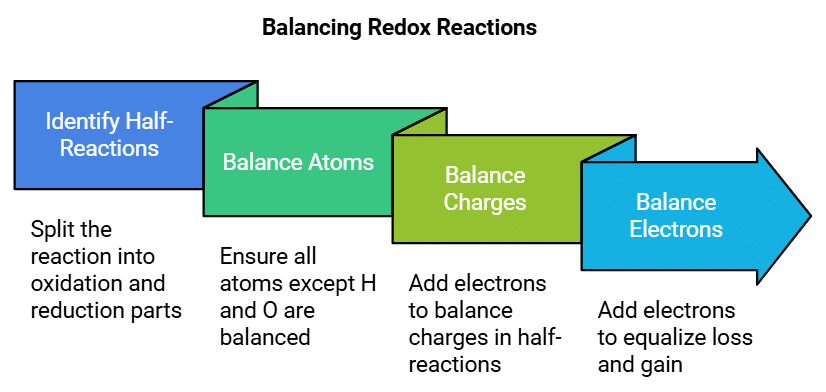
Balancing Redox Reactions using the Electron Transfer Method
Describes: The electron transfer method involves splitting the redox reaction into two half-reactions (oxidation and reduction), and then balancing them separately.
- Mnemonic: "Balance Atoms, Charge, Electrons."
- Balance: Start by balancing the atoms (except H and O).
- Charge: Balance the charges by adding electrons to either the oxidation or reduction half-reaction.
- Electrons: Finally, add electrons to balance the electron loss and gain between the two half-reactions.
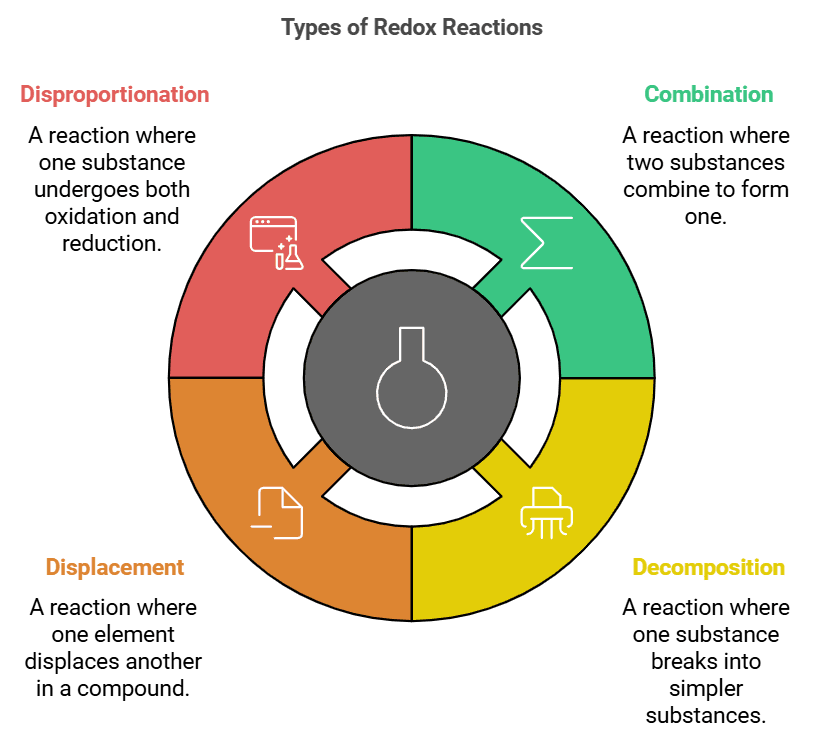
Types of Redox Reactions
Describes: Redox reactions can take place in different types of processes, such as combination, decomposition, displacement, and disproportionation.
- Mnemonic: "Silly Ducks Dance Deliberately."
- Silly: Synthesis (combination) reaction where two substances combine to form one.
- Ducks: Decomposition reaction where one substance breaks into two or more simpler substances.
- Dance: Displacement reaction where one element displaces another in a compound.
- Deliberately: Disproportionation reaction where one substance simultaneously undergoes both oxidation and reduction.
Anode and Cathode
Describes: In a redox reaction, the anode is where oxidation happens (electrons are lost), and the cathode is where reduction happens (electrons are gained).
- Mnemonic: "Don't PANIC"
- PA: Positive is Anode.
- NIC: Negative is Cathode.

Electron Flow in Galvanic Cells
Describes: In a galvanic (voltaic) cell, electrons flow from the anode to the cathode through an external circuit.
- Mnemonic: "Fat Cat."
- Fat: Electrons flow from Anode .
- Cat: to Cathode.
This helps remember the flow of electrons in a galvanic cell from anode to cathode.

With these mnemonics, you'll have a clearer understanding of Redox Reactions, making it easier to remember and apply concepts such as oxidation, reduction, electron transfer, and balancing redox reactions!
|
114 videos|263 docs|74 tests
|
FAQs on Mnemonics: Redox Reaction - Chemistry Class 11 - NEET
| 1. What are redox reactions and how do they work? |  |
| 2. How can I identify the oxidizing and reducing agents in a redox reaction? |  |
| 3. What is the electron transfer method for balancing redox reactions? |  |
| 4. What are the different types of redox reactions? |  |
| 5. What is the significance of anodes and cathodes in galvanic cells? |  |





















