NEET Exam > NEET Notes > Chemistry Class 11 > Mnemonics: Organic Chemistry : Some Basic Principles & Techniques
Mnemonics: Organic Chemistry : Some Basic Principles & Techniques | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Introduction |

|
| IUPAC Naming of Compounds |

|
| Carbocation and Carbanion |

|
| Types of Isomerism |

|
| Markovnikov’s Rule |

|
| Anti Markovnikov's Rule |

|
Introduction
Understanding the principles of organic chemistry can be challenging, but mnemonics offer an effective method to simplify and retain complex concepts, making them easier to recall.
IUPAC Naming of Compounds
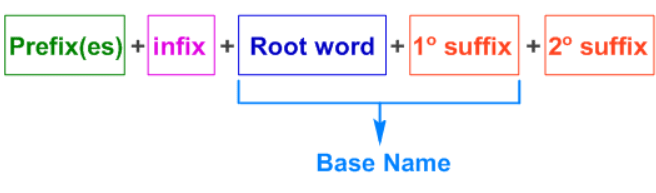
- Mnemonic: "Penny Wants Sweets First"
- Penny = Prefix (Substituents and their positions) . Start with the prefix for any substituents (halogens, alkyl groups, etc.) and their positions on the carbon chain.
- Wants = Word Root (Carbon chain length). After the prefix, identify the word root, which indicates the number of carbon atoms in the longest continuous chain (e.g., "meth-" for 1, "eth-" for 2, "prop-" for 3, etc.).
- Sweets = Suffix (Functional group). Finally, apply the suffix, which tells you the type of compound based on its functional group (e.g., -ane for alkane, -ene for alkene, -ol for alcohol, -al for aldehyde, etc.).
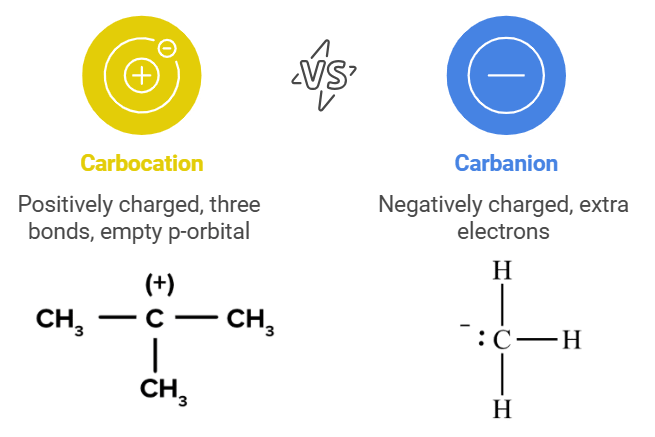
Carbocation and Carbanion
- A Carbocation is a positively charged species where a carbon atom has three bonds and an empty p-orbital.
- A Carbanion is a negatively charged species where a carbon atom has an extra pair of electrons.
- Mnemonic: "Cations Love Crowds, Anions Stay Alone."
- Cations: Carbocations are stabilized by more alkyl groups (tertiary > secondary > primary).
- Love Crowds: More alkyl groups stabilize the positive charge via hyperconjugation and induction.
- Anions: Carbanions are destabilized by alkyl groups (methyl > primary > secondary > tertiary).
- Stay Alone: Fewer alkyl groups provide more stability to the negative charge.
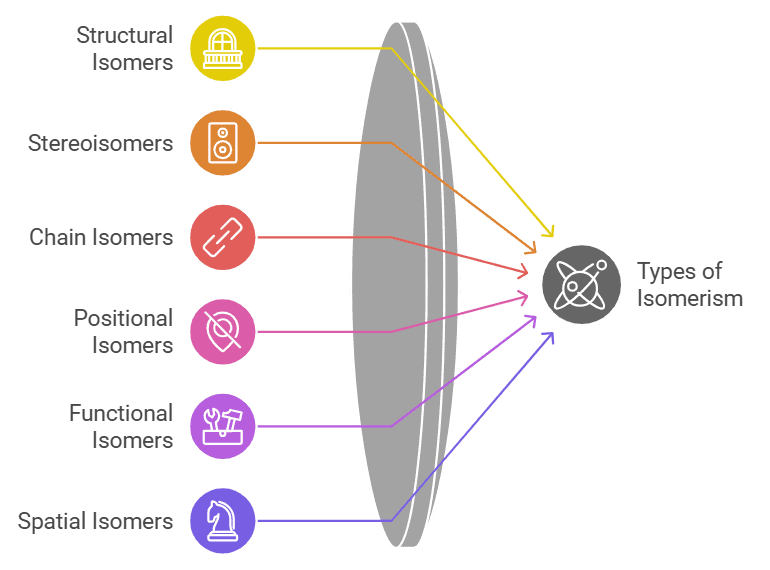
Types of Isomerism
Different types of isomers in organic chemistry.
- Mnemonic: "Smart Scientists Can Predict Fun Structures."
- Smart: Structural isomers.
- Scientists: Stereoisomers.
- Can: Chain isomers.
- Predict: Positional isomers.
- Fun: Functional isomers.
- Structures: Spatial (geometrical) isomers.
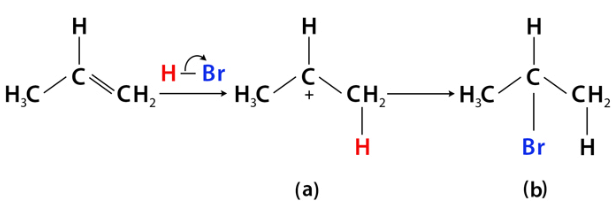
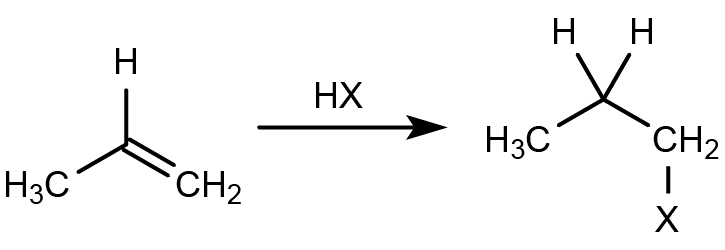
Markovnikov’s Rule
The rule for the addition of hydrogen halides to alkenes.
- Mnemonics: More Friends, More Hydrogen."
- More Friends: Carbon with more hydrogens gets more hydrogens.
- More Hydrogen: Hydrogen adds where hydrogens are already abundant.
Anti Markovnikov's Rule
In the presence of peroxide, the addition of hydrogen halides (like HBr) to alkenes occurs in such a way that the hydrogen atom attaches to the more substituted carbon, and the halogen attaches to the less substituted carbon.
- Mnemonic: "Anti Mark Prefers Poor Places."
- Anti: Anti-Markovnikov’s Rule.
- Mark: Opposite of Markovnikov’s Rule.
- Prefers: Peroxides initiate the reaction.
- Poor: Halogen prefers the less substituted carbon (electron-poor place).
- Places: Hydrogen bonds to the more substituted carbon.
Mnemonics serve as valuable tools in mastering organic chemistry, enhancing both comprehension and retention of key principles for practical application.
The document Mnemonics: Organic Chemistry : Some Basic Principles & Techniques | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Mnemonics: Organic Chemistry : Some Basic Principles & Techniques - Chemistry Class 11 - NEET
| 1. What is IUPAC naming and why is it important in organic chemistry? |  |
Ans.IUPAC naming (International Union of Pure and Applied Chemistry) is a systematic method for naming organic chemical compounds. It is important because it provides a unique and universally accepted name for each compound, which helps avoid confusion in communication among chemists. This system considers the structure of the compound and its functional groups, ensuring clarity in identifying and discussing chemical substances.
| 2. What are carbocations and carbanions, and how do they differ from each other? |  |
Ans.Carbocations are positively charged species that have a carbon atom with only six electrons in its valence shell, making it electron-deficient and reactive. In contrast, carbanions are negatively charged species with a carbon atom that has a complete octet of electrons, resulting in excess electron density. The key difference lies in their charge and electron configuration, which leads to different reactivity patterns in organic reactions.
| 3. What are the different types of isomerism in organic compounds? |  |
Ans.Isomerism in organic compounds can be categorized into two main types: structural isomerism and stereoisomerism. Structural isomerism occurs when compounds have the same molecular formula but different connectivity of atoms, leading to variations in their structure. Stereoisomerism, on the other hand, arises when compounds have the same molecular formula and connectivity but differ in the spatial arrangement of atoms, which includes geometric isomerism (cis/trans) and optical isomerism (enantiomers).
| 4. What is Markovnikov's Rule and how does it apply to addition reactions? |  |
Ans.Markovnikov's Rule states that when HX (where X is a halogen) is added to an alkene, the hydrogen atom will attach to the carbon with the greater number of hydrogen atoms already attached, while the halide will attach to the carbon with fewer hydrogen atoms. This rule helps predict the outcome of electrophilic addition reactions, ensuring that the most stable carbocation intermediate is formed during the reaction process.
| 5. What is Anti-Markovnikov's Rule and when does it apply? |  |
Ans.Anti-Markovnikov's Rule applies to certain addition reactions, particularly those involving peroxides. It states that when HX is added to an alkene in the presence of peroxides, the hydrogen will add to the carbon with fewer hydrogen atoms, and the halide will add to the carbon with more hydrogen atoms. This counterintuitive result is due to the formation of a radical intermediate, leading to the opposite outcome compared to Markovnikov's Rule.
Related Searches





















