NEET Exam > NEET Notes > Chemistry Class 12 > Mnemonics: D and F- Block
Mnemonics: D and F- Block | Chemistry Class 12 - NEET PDF Download
D-Block: Unveiling Elemental Mnemonics
The d-block, often referred to as the transition metals, boasts a rich tapestry of elements. Here are mnemonic devices to unlock their names:
First Transition Series (3d Series)
Elements: Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn
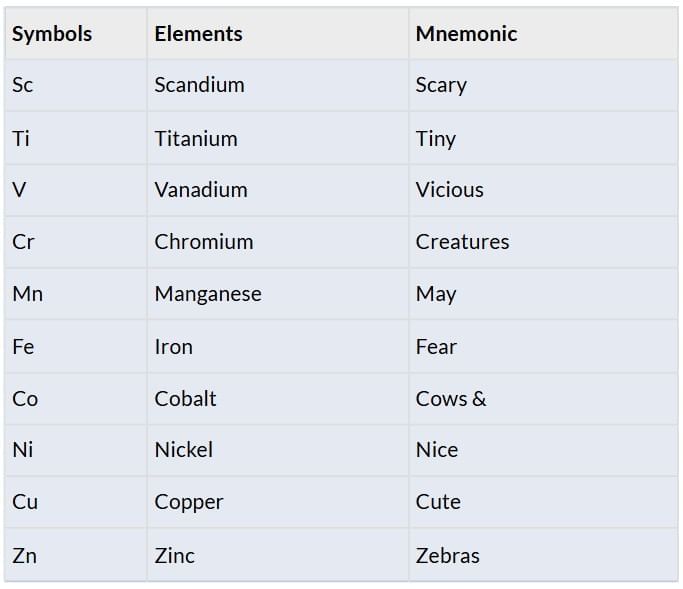
Second Transition Series (4d Series)
Elements: Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd
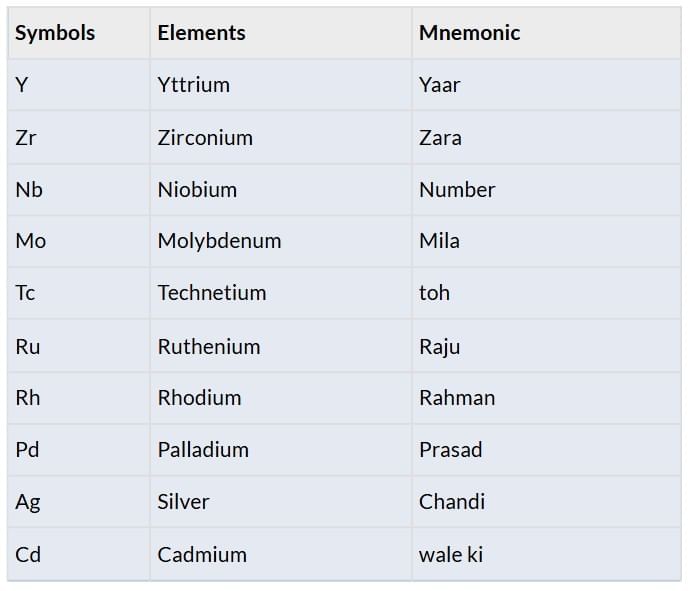
Third Transition Series (5d Series)
Elements: La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg
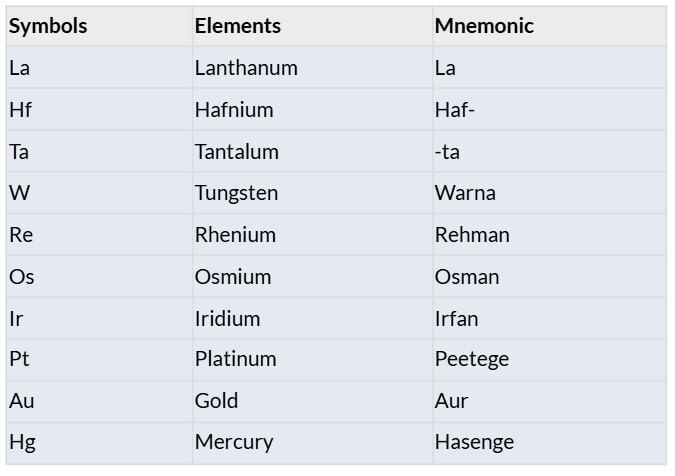
F-Block: Actinides and Lanthanides
The f-block, nestled discreetly at the bottom of the periodic table, is home to the actinides and lanthanides. These elements, often overlooked, play crucial roles in various scientific endeavors. Here’s a mnemonic aid to help remember them:
Lanthanides (4f Series)
Elements: La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu.
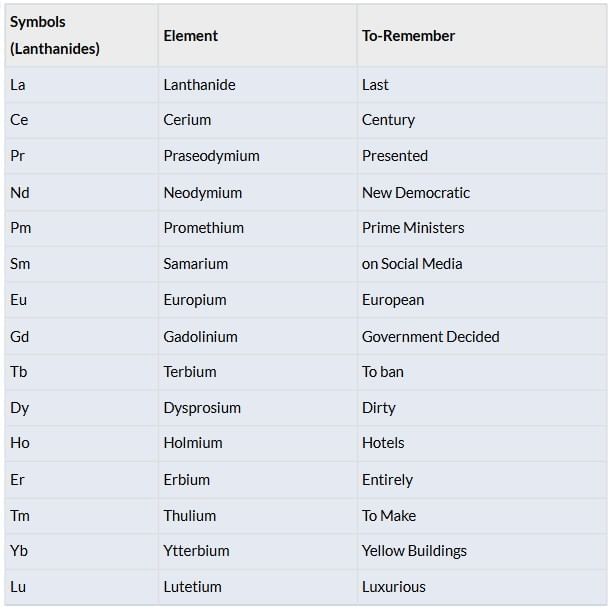
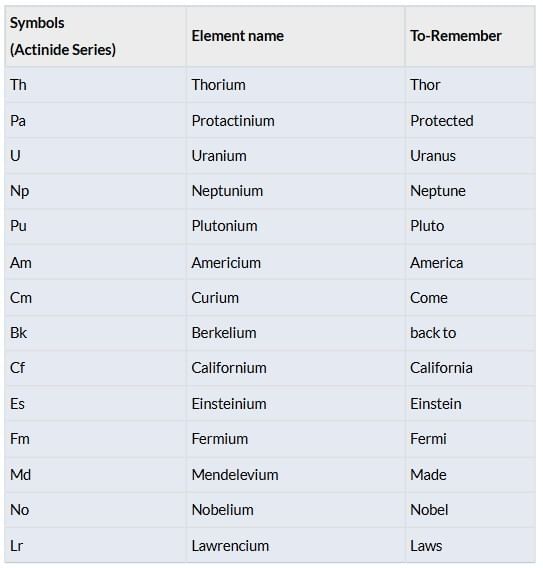
Actinides (5f Series)
Elements: Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr.
The document Mnemonics: D and F- Block | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|349 docs|78 tests
|
FAQs on Mnemonics: D and F- Block - Chemistry Class 12 - NEET
| 1. What are the characteristics of the first transition series (3d series) elements? |  |
Ans. The first transition series consists of elements from scandium (Sc) to zinc (Zn). These elements are characterized by the filling of the 3d subshell. They exhibit variable oxidation states, form colored compounds, and often act as good catalysts. Additionally, they possess magnetic properties and typically have high melting and boiling points.
| 2. How do the properties of the second transition series (4d series) differ from those of the first transition series? |  |
Ans. The second transition series includes elements from yttrium (Y) to cadmium (Cd) and involves the filling of the 4d subshell. Compared to the first series, these elements generally exhibit a greater range of oxidation states and a tendency to form more complex ions. They also tend to have higher melting and boiling points, and their compounds can be more stable due to stronger 4d-5s interactions.
| 3. What is the significance of lanthanides (4f series) in modern technology? |  |
Ans. Lanthanides, ranging from lanthanum (La) to lutetium (Lu), are crucial in modern technology due to their unique electronic properties. They are used in making strong permanent magnets, phosphors for LED lights, catalysts in petroleum refining, and in various electronic devices. Their ability to absorb and emit light makes them valuable in display technologies and lasers.
| 4. Can you explain the differences between lanthanides and actinides (5f series)? |  |
Ans. Lanthanides (4f series) and actinides (5f series) differ primarily in their electronic configurations and chemical properties. Lanthanides are generally more stable and less radioactive, while actinides, ranging from actinium (Ac) to lawrencium (Lr), are known for their radioactivity and more complex chemistry. Actinides can form compounds with a wider variety of oxidation states compared to lanthanides, which typically exhibit +3 oxidation states.
| 5. What are some effective mnemonics to remember the d and f block elements? |  |
Ans. One effective mnemonic for the first transition series (3d) is "Scary TiVans Crave Mn FeCo NiCu Zn," representing Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, and Zinc. For the lanthanides, you can use "Lazy Sam Prefers Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu" to remember the order of elements from Lanthanum to Lutetium. For actinides, "Acting ThUranium Neptunes Plutonium Americium Curium Berkelium Californium Einsteinium" can be used to memorize the series from Actinium to Einsteinium.
Related Searches

















