Mnemonics: Kinetic Theory | Physics Class 11 - NEET PDF Download
Understanding gas behavior can sometimes feel challenging, but mnemonics make it simple and fun! This document provides creative and relatable mnemonics for gas equations & laws, helping you remember their principles and formulas effortlessly. Dive in to connect these concepts with everyday examples, making your learning process smooth and enjoyable!
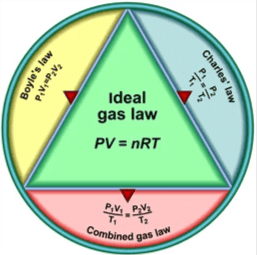
1. Ideal Gas Equation
Mnemonic: "Pressure Volume Takes UGC Moles."
- Pressure : Pressure (P)
- Volume : Volume (V)
- Takes : Temperature (T)
- UGC : Universal Gas Constant (R)
- Moles : Number of moles (n)
Formula: PV = nRT
Pressure × Volume = Number of moles × Gas constant × Temperature.
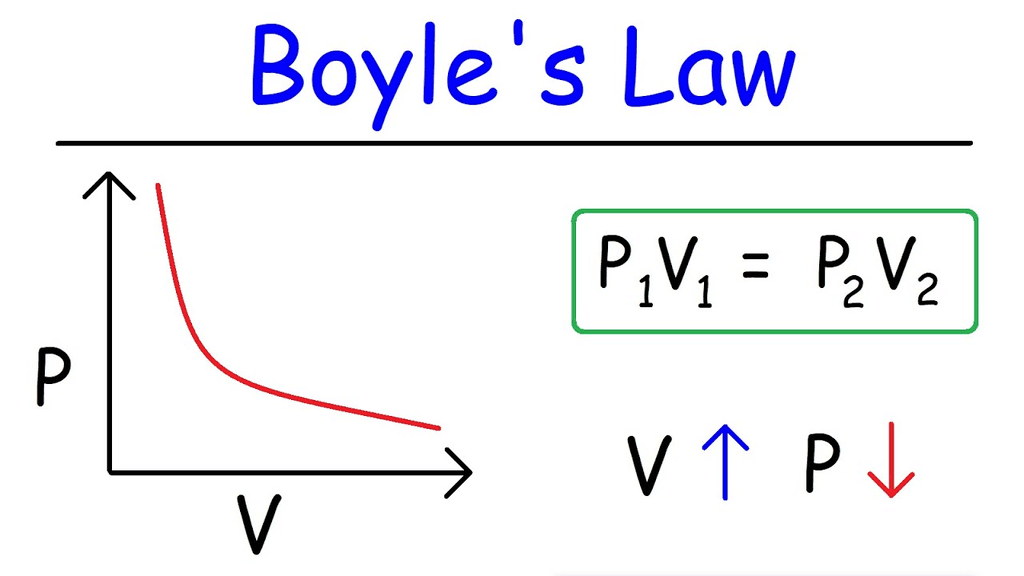
2. Boyle's Law
Mnemonic: "Boyle's Volume dislikes Pressure."
Mnemonic Explanation:
- Boyle's Volume: When the volume of a gas increases, its particles have more space to move around, leading to fewer collisions with the container walls.
- Dislikes Pressure: Fewer collisions mean a decrease in pressure.
Boyle's Law states:
For a given mass of gas at constant temperature, the pressure of the gas is inversely proportional to its volume. Mathematically:= constant.
3. Charles' Law
Mnemonic: "Charles' Volume Likes Heat."
Mnemonic Explanation:
- Charles' Volume: Think of a balloon as an example of gas volume.
- Likes Heat: As the temperature of the gas inside the balloon increases, the gas molecules move faster, and the balloon expands (volume increases).
Charles' Law states:
For a given mass of gas at constant pressure, the volume of the gas is directly proportional to its absolute temperature. Mathematically:
= constant.
This means when the temperature of a gas increases, its volume also increases, provided pressure remains constant.
This mnemonic links the observable behavior of a gas-filled balloon to Charles' Law: higher the temperature, larger the volume. It’s an easy and relatable way to understand the principle!
|
96 videos|367 docs|98 tests
|
FAQs on Mnemonics: Kinetic Theory - Physics Class 11 - NEET
| 1. What is the Ideal Gas Equation and what do the variables represent? |  |
| 2. How does Boyle's Law relate pressure and volume of a gas? |  |
| 3. What is Charles' Law and how does it apply to the behavior of gases? |  |
| 4. What are the key assumptions of the Kinetic Theory of Gases? |  |
| 5. How can mnemonics help in remembering gas laws and equations? |  |