DPP for NEET: Daily Practice Problems, Ch: Some Basic Concepts of Chemistry | Chemistry Class 11 PDF Download
PART-I (Single Correct MCQs)
Q1: The weight of NaCl decomposed by 4.9g of H2SO4, if 6 g of sodium hydrogen sulphate and 1.825 g of HCI, were produced in the reaction is:
(a) 6.921 g
(b) 4.65 g
(c) 2.925 g
(d) 1.4g
Q2: 6.02 x 1020 molecules of urea are present in 100 ml of its solution. The concentration of urea solution is
(a) 0.02 M
(b) 0.01 M
(c) 0.001 M
(d) 0.1 M
(Avogadro constant, NA = 6.02 x 1023 mol-1)
Q3: If we consider that 1/6, in place of 1/12, mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of the substance
(a) be a function of the molecular mass of the substance
(b) remain unchanged
(c)increase two fold
(d) decrease twice
Q4: How many moles of magnesium phosphate, will contain 0.25 mole of oxygen atoms?
(a) 1.25 × 10–2
(b) 2.5 × 10–2
(c) 0.02
(d) 3.125 × 10–2
Q5: A gas occupies a volume of 300 cc at 27°C and 620 mm pressure. The volume of gas at 47°C and 640 mm pressure is:
(a) 260 cc
(b) 310 cc
(c) 390 cc
(d) 450 cc
Q6: Haemoglobin contains 0.33% of iron by weight. The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe = 56) present in one molecule of haemoglobin is
(a) 6
(b) 1
(c) 2
(d) 4
Q7: In the reaction,
2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl-(aq) + 3H2(g)
(a) 11.2 L H2(g) at STP is produced for every mole HCl(aq) consumed
(b) 6 L HCl(aq) is consumed for every 3 L H2(g) produced
(c) 33.6 L H2(g) is produced regardless of temperature and pressure for every mole A1 that reacts
(d) 67.2 H2(g) at STP is produced for every mole A1 that reacts.
Q8: The concentrated sulphuric acid that is peddled commercial is 95% H2SO4 by weight. If the density of this commercial acid is 1.834 g cm–3, then find the molarity of this solution.
(a) 17.8 M
(b) 12.0 M
(c) 10.5 M
(d) 15.7 M
Q9: What is the mass of precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution ?
(Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5)
(a) 28 g
(b) 3.5 g
(c) 7 g
(d) 14 g
10: Number of valence electrons in 4.2 gram of N3– ion is
(a) 4.2 NA
(b) 0.1 NA
(c) 1.6 NA
(d) 3.2 NA
Q11: A transition metal M forms a volatile chloride which has a vapour density of 94.8. If it contains 74.75% of chlorine the formula of the metal chloride will be
(a) MCl3
(b) MCl2
(c) MCl4
(d) MCl5
Q12: A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g. of CO2. The empirical formula of the hydrocarbon is :
(a) C2H4
(b) C3H4
(c) C6H5
(d) C7H8
Q13: Following is the composition of a washing soda sample :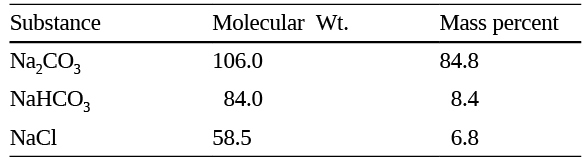
On complete reaction with excess HCl, one kilogram of the washing soda will evolve:
(a) 9 mol of CO2
(b) 16 mol of CO2
(c) 17 mol of CO2
(d) 18 mol of CO2
Q14: Arrange the numbers in increasing no. of significant figures.
0.002600, 2.6000, 2.6, 0.260
(a) 2.6 < 0.260 < 0.002600 < 2.6000
(b) 2.6000 < 2.6 < 0.002600 < 0.260
(c) 0.260 < 2.6 < 0.002600 < 2.6000
(d) 0.002600 < 0.260 < 2.6 < 2.6000
Q15: Dissolving 120 g of a compound (mol. wt. 60) in 1000 g of water gave a solution of density 1.12 g/mL. The molarity of the solution is:
(a) 1.00 M
(b) 2.00 M
(c) 2.50 M
(d) 4.00 M
Q16: A gaseous compound of nitrogen and hydrogen contains 12.5% (by mass) of hydrogen. The density of the compound relative to hydrogen is 16. The molecular formula of the compound is:
(a) NH2
(b) N3H
(c) NH3
(d) N2H4
Q17: The amount of BaSO4 formed upon mixing 100 mL of 20.8% BaCl2 solution with 50 mL of 9.8% H2SO4 solution with 50 mL of 9.8% H2SO4 solution will be:
(Ba = 137, Cl = 35.5, S = 32, H = 1 and O = 16)
(a) 23.3 g
(b) 11.65 g
(c) 30.6 g
(d) 33.2 g
Q18: Number of moles of KMnO4 required to oxidize one mole of Fe(C2O4) in acidic medium is
(a) 0.167
(b) 0.6
(c) 0.2
(d) 0.4
Q19: A gas mixture of 3 litres of propane (C3H8) and butane (C4H10) on complete combustion at 25° C produced 10 litre CO2. Find out the composition of gas mixture (Propane : Butane)
(a) 2 : 1
(b) 1 : 2
(c) 1.5 : 1.5
(d) 0.5 : 2.5
Q20: An organic compound contains 49.3% carbon, 6.84% hydrogen and its vapour density is 73. Molecular formula of the compound is :
(a) C3H5O2
(b) C4H10O2
(c) C6H10O4
(d) C3H10O2
Q21: 1022 molecules are removed from 200 mg of CO2. Calculate the moles of CO2 left.
Q22: What volume of hydrogen gas, at 273 K and 1 atm. pressure will be consumed in obtaining 21.6 g of elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen ?
Q23: What will be the density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29% H2SO4 (molar mass = 98 g mol–1) by mass?
Q24: 2 g of a mixture of CO and CO2 on reaction with excess I2O5 produced 2.54 g of . What will be the mass % of CO2 in the original mixture ?
Q25: What is the weight of oxygen required for the complete combustion of 2.8 kg of ethylene ?
|
114 videos|263 docs|74 tests
|





















