EmSAT Achieve Exam > EmSAT Achieve Notes > Physics for EmSAT Achieve > Mind Map: Atomic Physics
Mind Map: Atomic Physics | Physics for EmSAT Achieve PDF Download
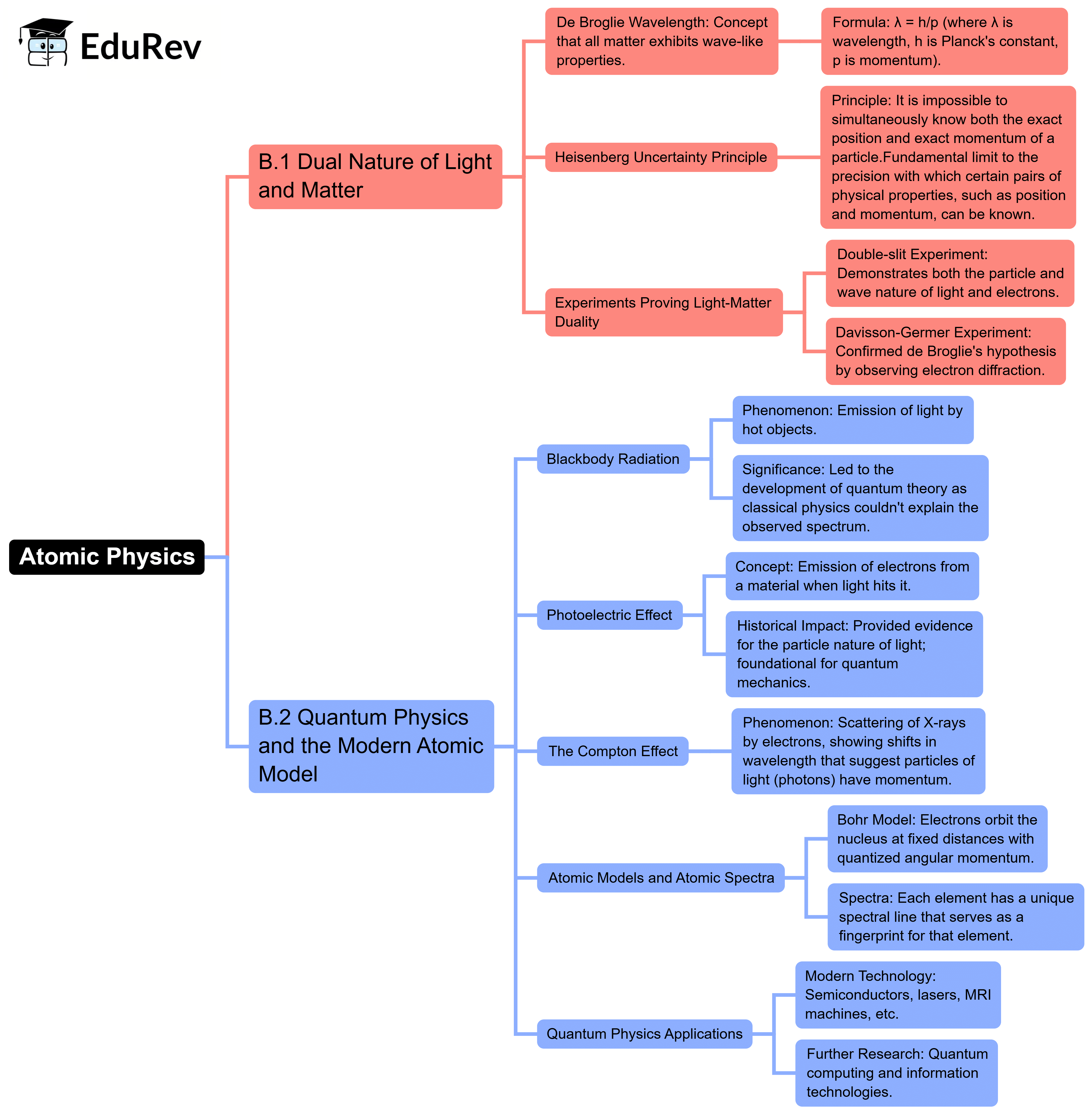
The document Mind Map: Atomic Physics | Physics for EmSAT Achieve is a part of the EmSAT Achieve Course Physics for EmSAT Achieve.
All you need of EmSAT Achieve at this link: EmSAT Achieve
|
209 videos|327 docs|212 tests
|
FAQs on Mind Map: Atomic Physics - Physics for EmSAT Achieve
| 1. What is atomic physics and why is it important? |  |
Ans.Atomic physics is the field of physics that studies atoms as isolated systems comprising electrons and a nucleus. It plays a crucial role in understanding fundamental concepts such as atomic structure, quantum mechanics, and the behavior of matter at a microscopic level. This knowledge has applications in various fields, including chemistry, materials science, and nuclear engineering.
| 2. What are the main components of an atom? |  |
Ans.An atom consists of three primary components: protons, neutrons, and electrons. Protons are positively charged particles found in the nucleus, neutrons are neutral particles also located in the nucleus, and electrons are negatively charged particles that orbit the nucleus in various energy levels. The arrangement of these particles determines the chemical properties of an element.
| 3. How do atomic models evolve over time? |  |
Ans.Atomic models have evolved significantly over the years. Early theories, such as Dalton's solid sphere model, were later refined by Thomson’s plum pudding model, which introduced the idea of electrons as components of the atom. Rutherford’s gold foil experiment led to the discovery of the nucleus, and Bohr’s model introduced quantized energy levels. The current quantum mechanical model provides a more accurate description of electron behavior and atomic structure.
| 4. What is the significance of isotopes in atomic physics? |  |
Ans.Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. They play a significant role in atomic physics because they can exhibit different physical and chemical properties. Isotopes are essential in various applications, including radiometric dating, medical imaging, and nuclear reactors, allowing scientists to study processes and phenomena related to atomic behavior.
| 5. What role does quantum mechanics play in atomic physics? |  |
Ans.Quantum mechanics is fundamental to atomic physics as it provides the framework for understanding the behavior of particles at the atomic and subatomic levels. It explains phenomena such as electron configuration, energy quantization, and wave-particle duality. Quantum mechanics has led to significant advancements in technology, including semiconductors, lasers, and quantum computing, which rely on principles derived from atomic physics.
Related Searches













