Class 10 Exam > Class 10 Notes > Physics Class 10 ICSE > Revision Notes: Calorimetry
Revision Notes: Calorimetry | Physics Class 10 ICSE PDF Download
Concept of Heat and Temperature
- When two bodies at different temperatures are kept in contact, heat flows from the body at a higher temperature to the body at a lower temperature.
- The measurement of heat is called calorimetry.
Units of Heat
- The SI unit of heat is joule (J). The other most commonly used unit of heat is calorie (cal).
- The heat energy required to raise the temperature of 1 g of water through 1°C is known as calorie.
- The unit calorie is related to the SI unit joule as follows:
1 calorie (or 1 cal) = 4·186 J or 4·2 J
Factors affecting the quantity of heat required to raise the temperature of a body
- The quantity of heat energy required to raise the temperature of a body depends on three factors:
i. Mass of the body
ii. Nature of the material of the body
iii. Rise in temperature of the body
Few observations through experiments
i. The amount of heat energy required is found to be directly proportional to the mass of the substance.
ΔQ ∝ m
ii. The amount of heat energy required is found to be directly proportional to the rise in temperature.
ΔQ ∝ ΔT
Therefore,
ΔQ ∝ cmΔT
where c is the constant of proportionality and is called the specific heat capacity of the substance. It is the characteristic of the substance.
Heat Capacity
- The heat capacity of a body is the amount of heat energy required to raise its temperature by 1°C or 1 K.
- It is denoted by the symbol C′.
C' = (Amount of heat energy supplied/Rise in temperature) = ΔQ/ΔT - The SI unit of heat capacity is joule per Kelvin (or J K−1). It is also written as joule per degree C (or J °C−1).
- The other common units of heat capacity are calorie °C−1 (or cal K−1) and kilo-calorie °C−1 (or kilo-calorie K−1).
1 kilo calorie °C-1 = 1000 calorie °C-1
And
1 cal K-1 = 4.2 J K-1
Specific Heat Capacity
- The specific heat capacity of a substance (or a body) is defined as the heat capacity per unit mass of that body.
C = Heat capacity of the body C'/Mass of the body m - The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of a unit mass of that substance through 1°C (or 1 K).
c = (Amount of heat energy supplied/(Mass x Rise in temperature))
= ΔQ/(m x ΔQ) - The SI unit of specific heat capacity is joule per kilogram per Kelvin (or J kg−1 K−1) or joule per kilogram per degree Celsius (or J kg−1 °C−1).
Calorimeter
- A calorimeter is a cylindrical vessel which is used to measure the amount of heat gained or lost by a body when it is mixed with another body.
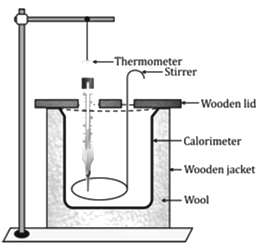
- It is made up of thin copper sheets because
i. Copper is a good conductor of heat, so the vessel soon acquires the temperature of its contents.
ii. Copper has low specific heat capacity, so the heat capacity of the calorimeter is low and the amount of heat energy taken by the calorimeter from its contents to acquire the temperature of its contents is negligible.
Principle of Calorimetry
- When a hot body is mixed (or is kept in contact) with a cold body, heat energy passes from the hot body to the cold body, till both the bodies attain the same temperature. If no heat energy is exchanged with the surroundings, i.e. if the system is fully insulated, then
Heat energy lost by the hot body = Heat energy gained by the cold body - This is called the principle of mixtures or the principle of calorimetry.
m1c1(T1 - T) = m2c2(T - T2)
Measurement of Specific Heat Capacity of a Solid
Let c J kg−1 K−1 be the specific heat capacity of the solid submerged in a calorimeter, while Cc J kg−1 K−1 and Cw J kg−1 K−1 be the specific heat capacities of the material of the calorimeter and of water, respectively.
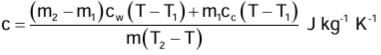
Measurement of Specific Heat Capacity of a Liquid
In this case, we take a solid of known specific heat capacity c. The solid must not react chemically with the given liquid whose specific heat capacity is to be determined. The liquid is put in the calorimeter in place of water. If cL J kg−1 K−1 is the specific heat capacity of liquid, then
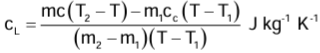
Measurement of Specific Heat Capacity: Electrical Method
- Heat energy is supplied by an electric heater of known power P. The rise in temperature ΔT in time t for a known mass m of the given substance is noted.
- Assuming that there is no loss of heat, the energy supplied by the heater (= Pt) is equal to the energy used (= mcΔT) in raising the temperature of the substance. Thus,
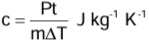
- This method is suitable for good conductors of heat such as copper, silver, aluminium and mercury because they get heated uniformly.
Consequence of High Specific Heat Capacity of Water
- The climate near the seashore is moderate.
- Hot water bottles are used for fomentation: Water does not cool quickly due to its large specific heat capacity, so a hot water bottle provides heat energy for fomentation for a longer period.
- Water is used as an effective coolant.
- Farmers fill their fields with water to protect the crops from frost.
- All plants and animals have a high content of water in their bodies.
Change of Phase
- The process of change from one state to another at a constant temperature is called the change of phase.
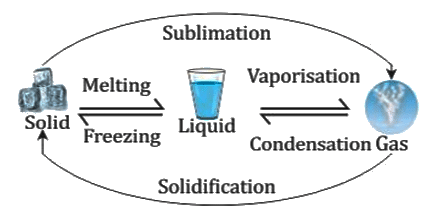
Melting and fusion
- The change from solid to liquid on heating at a constant temperature is called melting.
- The constant temperature at which a solid changes to liquid is called the melting point of the solid.
- The reverse change from liquid to solid with the rejection of heat at a constant temperature is called freezing (or fusion), and the temperature at which a liquid freezes to solid is called its freezing point.
Exchange of Heat during the Change of Phase
i. Change of phase from ice to water
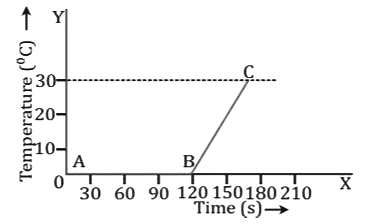
- The temperature of ice remains constant (equal to 0°C) in the part AB till the whole ice melts. The heat supplied during this time is used in melting the ice. After this, the temperature of water formed by melting of ice begins to rise in the part BC. The constant temperature at which the ice melts is the melting point of ice.
ii. Change of phase from solid to liquid and liquid to solid in naphthalene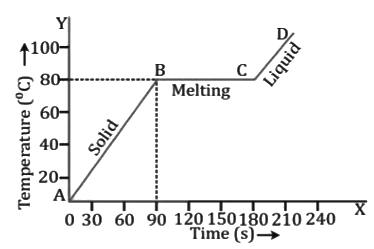 Heating Curve for Naphthalene
Heating Curve for Naphthalene
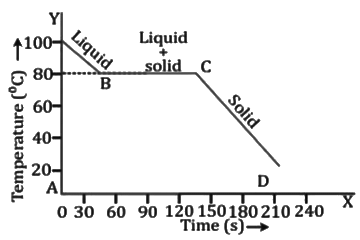
Cooling Curve for Naphthalene
- The temperature does not change when the change of phase occurs, although heat energy is absorbed during melting and heat energy is rejected while freezing.
- The melting point of naphthalene is 80°C. At point C, the entire solid has completely melted and the temperature has started increasing once again as shown by the part CD of the heating curve.
- During freezing, the temperature remains constant at 80°C, although naphthalene is losing heat energy continuously by radiating it to the surrounding. Thus, the freezing point of naphthalene is 80°C. Part CD indicates that the temperature of solid is decreasing.
- Thus, the melting point and the freezing point are the same.
(a) Effect of pressure on the melting point
- The melting point of the substances which contract on melting (e.g. ice) decreases with an increase in pressure.
- On the other hand, the melting point of the substances (e.g. wax, lead) which expand on melting increases with an increase in pressure.
(b) Effect of impurities on the melting point
- The melting point of a substance decreases by the presence of impurities in it. Thus, in making a freezing mixture, salt is added to ice. The freezing mixture is used in preparing 'kulphies'.
Vaporisation and Boiling
Heating curve for water (absorption of heat energy during vaporisation)
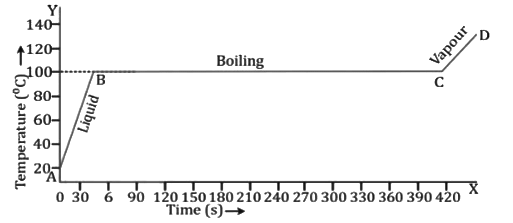
Change in Volume on Boiling
- All liquids expand on boiling.
- The boiling point of a liquid depends on (a) the pressure on its surface and (b) the presence of impurities in it.
(a) Effect of pressure on the boiling point
- The boiling point of a liquid increases with an increase in pressure and decreases with a decrease in pressure.
(b) Effect of impurities on the boiling point
- The boiling point of a liquid increases by the addition of impurities to it. If a little common salt is added to water, the water boils at a temperature higher than 100°C. Thus, the addition of salt makes cooking faster because water provides sufficient heat energy before it boils.
Latent Heat and Specific Latent Heat
- The heat energy exchanged in change of phase is not externally manifested by any rise or fall in temperature, and hence, it is considered to be hidden in the substance and is called the latent heat. Latent heat when expressed for unit mass of the substance is called the specific latent heat.
- Specific latent heat is denoted by the symbol L. Thus, specific latent heat
L = Heat energy exchanged for the change of phase/Mass
L = Q/m
Specific Latent Heat of Melting and Fusion
- The specific latent heat of melting of a substance is defined as the heat energy required to convert a unit mass of a substance from solid to liquid without a change in temperature.
- The specific latent heat of fusion of a substance is the heat energy released when a unit mass of a substance changes from liquid to solid without a change in temperature.
Unit of specific latent heat
The SI unit of specific latent heat is J kg-1.
Other common units are cal g-1 and kilo-calorie kg-1.
1 kilo-cal kg-1 = 1 cal g-1
1 cal g-1 = 4.2 J g-1
1 cal g-1 = 4.2 x 103 J kg-1
Consequences of High Specific Latent Heat of Fusion of Ic
- Snow on mountains does not melt all at once.
- Water in lakes and ponds in cold countries does not freeze all at once.
- Drinks get cooled more quickly by adding pieces of ice at 0°C than the ice-cold water at 0°C.
- When ice in a frozen lake starts melting, its surrounding becomes very cold.
- It is generally more cold after a hail storm (when ice melts) than during or before the hail storm.
Measurement of Specific Latent Heat of Ice
- Electrical method: Heat energy is supplied for a known time t by an electric heater of known power P to melt the ice at 0°C.
Specific latent heat of ice L = Pt/m - Method of mixture: Heat energy given by water and calorimeter = Heat energy taken by ice on melting and then by melted ice in rise of temperature
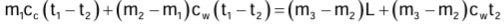
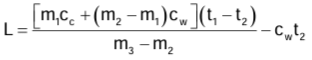
The document Revision Notes: Calorimetry | Physics Class 10 ICSE is a part of the Class 10 Course Physics Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
28 videos|121 docs|14 tests
|
FAQs on Revision Notes: Calorimetry - Physics Class 10 ICSE
| 1. What is the difference between heat and temperature? |  |
Ans. Heat is the energy transferred between systems or objects with different temperatures, while temperature is a measure of the average kinetic energy of the particles in a substance. Heat is a form of energy, whereas temperature indicates how hot or cold an object is.
| 2. How is specific heat capacity measured for a solid? |  |
Ans. The specific heat capacity of a solid can be measured using a calorimeter. The solid is heated to a known temperature and then placed in a calorimeter containing a known mass of water at a specific initial temperature. By measuring the temperature change of the water and using the formula \( Q = mc\Delta T \), where \( Q \) is the heat gained or lost, \( m \) is the mass, \( c \) is the specific heat capacity, and \( \Delta T \) is the change in temperature, the specific heat capacity of the solid can be calculated.
| 3. Why is water known for having a high specific heat capacity? |  |
Ans. Water has a high specific heat capacity due to the hydrogen bonds between its molecules. These bonds require a significant amount of energy to break, which means water can absorb a lot of heat without a large increase in temperature. This property helps regulate temperatures in the environment and in biological systems.
| 4. What is the principle of calorimetry? |  |
Ans. The principle of calorimetry is based on the conservation of energy, which states that the heat lost by a hotter object must equal the heat gained by a cooler object when they are brought into thermal contact. This allows the calculation of heat transfer between substances and the determination of specific heat capacities.
| 5. What happens during the melting phase change? |  |
Ans. During the melting phase change, a solid absorbs heat energy, which causes its particles to gain kinetic energy and move more freely. This process breaks the intermolecular forces that hold the particles in the solid state, allowing the substance to transition into the liquid state at its melting point.
Related Searches
















