Grade 10 Exam > Grade 10 Notes > Technical Science for Grade 10 > Revision Notes: Chemical Bonding - Ionic Compounds and Covalent Compounds
Revision Notes: Chemical Bonding - Ionic Compounds and Covalent Compounds | Technical Science for Grade 10 PDF Download
| Table of contents |

|
| Chemical Bonding |

|
| Electrovalent (or Ionic) Bond |

|
| Covalent Bond |

|
| Coordinate Bond |

|
| Redox Reaction |

|
Chemical Bonding
A chemical bond is defined as the force of attraction between any two atoms in a molecule to maintain stability.
Noble Gases
- Have stable electronic configuration, i.e. their outermost shell is complete.
- They have 2 electrons in the outermost shell or 8 electrons in the outermost shell.
- They do not lose, gain or share electrons and are inert or unreactive.
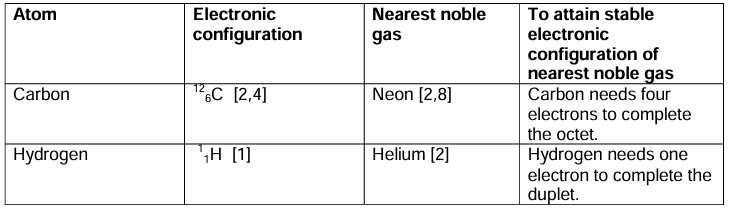
Atoms of Elements – Other than Noble Gases
- Have unstable electronic configuration, i.e. their outermost shell is incomplete.
- They can lose, gain or share electrons and are chemically reactive.
Reasons for Chemical Bonding
- The driving force for atoms to combine is related to the tendency of each atom to attain stable electronic configuration of the nearest inert noble gas.
- For an atom to achieve stable electronic configuration, it must have
Two electrons in the outermost shell (nearest noble gas He) – Duplet rule
Eight electrons in the outermost shell (all noble gases other than He) – Octet rule
Methods for achieving Chemical Bonding
There are three methods in which atoms can achieve a stable configuration:
- Electrovalent bond
- Covalent bond
- Coordinate bond
Electrovalent (or Ionic) Bond
- Ionic bond: The chemical bond formed between two atoms by transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
- Ionic compound: The chemical compound formed as a result of transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
- Electrovalency: The number of electrons donated or accepted by the valence shell of an atom of an element so as to achieve stable electronic configuration is called electrovalency.
Conditions for the formation of an Ionic Bond
- Ionisation potential (IP): Lower the value of IP of a metallic atom, greater the ease of formation of the cation.
- Electron affinity: Higher the value of EA of a non-metallic atom, greater the ease of formation of the anion.
- Electronegativity: Larger the differences in electronegativity between the combining atoms, greater the ease of electron transfer.
Formation of electrovalent compounds
Formation of Sodium Chloride
- Ionic equation
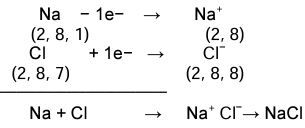
- Electron dot structural diagram
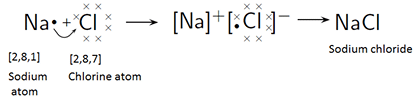
Covalent Bond
- Covalent bond: The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of non-metallic elements.
- Covalent compound: The chemical compound formed due to mutual sharing of electrons between the given pairs of atoms, thereby forming a covalent bond between them.
- Covalency: The number of electron pairs which an atom shares with one or more atoms of the same kind or different kind to achieve stable electronic configuration is called covalency.
- Non-polar covalent compounds: Covalent compounds are said to be non-polar when the shared pair of electrons are equally distributed between the two atoms.
Examples: H2, Cl2, O2, N2, CH4, CCl4 - Polar covalent compounds: Covalent compounds are said to be polar when a shared pair of electrons are unequally distributed between the two atoms.
Examples: H2O, NH3, HCl
Conditions for formation of covalent compound
Ionisation potential, electron affinity and electronegativity: High between both the atoms.
Electronegativity difference: Should be negligible between the two combining atoms.
Formation of methane molecule – Non-polar covalent compound
One atom of carbon shares four electron pairs, one with each of the four atoms of hydrogen.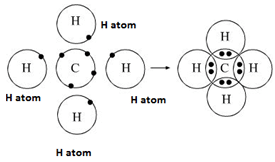
Coordinate Bond
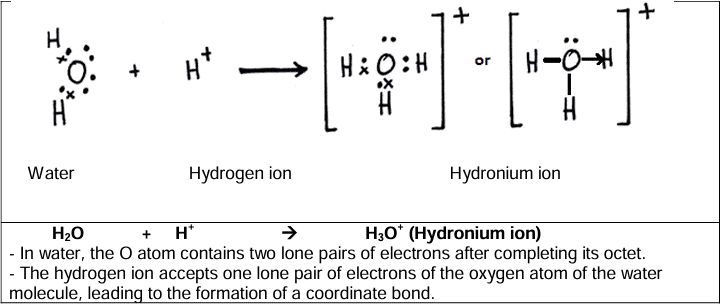
The bond formed between two atoms by sharing a pair of electrons provided entirely by one of the combining atoms but shared by both is called a coordinate bond or dative bond.Examples: Ammonium ion (NH4 +), hydronium ion (H3O+)
A coordinate bond has properties of both covalent and ionic bonds. So, it is also called a co-ionic bond.
- Lone pair of electrons: A pair of electrons which is not shared with any other atom is known as a lone pair of electrons. It is provided to the other atom for the formation of a coordinate bond.
- Conditions for the formation of coordinate bond
- One of the two atoms must have at least one lone pair of electrons. Examples: Ammonia (NH3), water (H2O)
- Another atom should be short of at least one lone pair of electrons. Example: Hydrogen ion (H+)
- Formation of hydronium ion [H3O+]
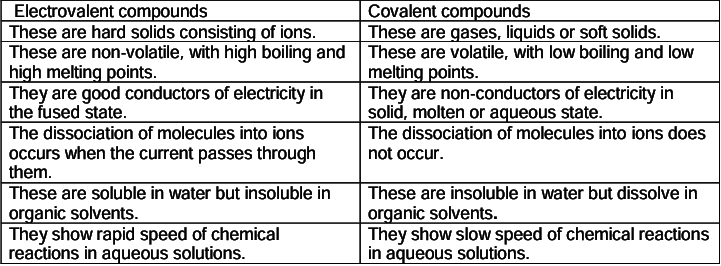
- Properties and comparison of electrovalent and covalent compounds
Redox Reaction
- Oxidation: When an atom or ion loses an electron or electrons, oxidation takes place.
- Reduction: When an atom or ion gains an electron or electrons, reduction takes place.
- Oxidising agents: The atom or ion which gains an electron or electrons is an oxidising agent.
- Reducing agents: The atom or ion which loses an electron or electrons is a reducing agent.
- Redox reaction: A chemical reaction in which the loss and gain of electrons take place simultaneously is called a redox reaction.
Example: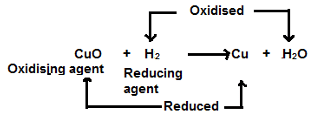
The document Revision Notes: Chemical Bonding - Ionic Compounds and Covalent Compounds | Technical Science for Grade 10 is a part of the Grade 10 Course Technical Science for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
1 videos|77 docs|5 tests
|
FAQs on Revision Notes: Chemical Bonding - Ionic Compounds and Covalent Compounds - Technical Science for Grade 10
| 1. What are noble gases and why are they considered inert? |  |
Ans.Noble gases are a group of elements in Group 18 of the periodic table, including helium, neon, argon, krypton, xenon, and radon. They are considered inert because they have a full valence shell of electrons, making them stable and unlikely to react with other elements to form compounds.
| 2. What are the main reasons for chemical bonding between atoms? |  |
Ans.The main reasons for chemical bonding include the desire of atoms to achieve a stable electron configuration, often resembling that of noble gases. Atoms bond to lower their potential energy, achieve full outer electron shells (octet rule), and enhance stability by forming compounds.
| 3. What conditions are necessary for the formation of an ionic bond? |  |
Ans.Ionic bonds form under conditions where there is a significant difference in electronegativity between the two atoms involved. Typically, this occurs between metals and nonmetals. The metal atom donates electrons to become positively charged, while the nonmetal atom accepts electrons to become negatively charged, resulting in electrostatic attraction.
| 4. How do covalent compounds form, and what conditions are required for their formation? |  |
Ans.Covalent compounds form when two nonmetal atoms share electrons to achieve a full outer shell. Conditions necessary for their formation include the presence of atoms with similar electronegativities and the overlap of their atomic orbitals, allowing for the sharing of electron pairs.
| 5. What distinguishes ionic compounds from covalent compounds? |  |
Ans.Ionic compounds are formed through the transfer of electrons from one atom to another, resulting in charged ions that attract each other, while covalent compounds are formed through the sharing of electrons between atoms. Ionic compounds typically have high melting and boiling points and conduct electricity when dissolved in water, whereas covalent compounds usually have lower melting and boiling points and may not conduct electricity.
Related Searches




















