Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Revision Notes: Analytical Chemistry : Uses of Ammonium Hydroxide and Sodium Hydroxide
Revision Notes: Analytical Chemistry : Uses of Ammonium Hydroxide and Sodium Hydroxide | Chemistry Class 10 ICSE PDF Download
Analytical Chemistry – Uses of Ammonium Hydroxide and Sodium Hydroxide
Introduction
The determination of the chemical components in a given sample is called analysis.
Types of Analysis
- Qualitative Analysis: It involves the identification of unknown substances.
- Quantitative Analysis: It involves the identification of the composition of a mixture.
Reagents
- Reagent: It is a substance which reacts with another substance.
- Alkalis are important laboratory reagents.
- Sodium hydroxide and ammonium hydroxide are the most commonly used alkalis, which give characteristic tests with various metal cations, and thus, a cation can be identified.
Colours of Salts and their Solutions
Salts of representative elements (normal elements), i.e. the elements of Group IA to Group VII A are generally colourless.
Salts of transition elements, i.e. salts of elements of Group IB to VIIB and Group VIII are generally coloured.
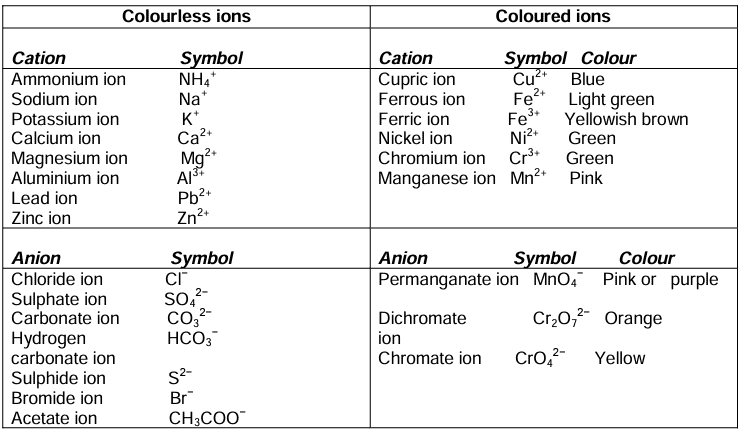
Precipitation
It is the process of formation of an insoluble solid when solutions are mixed. The solid thus formed is known as a precipitate.
Action of Sodium Hydroxide Solution on Metallic Salt Solutions
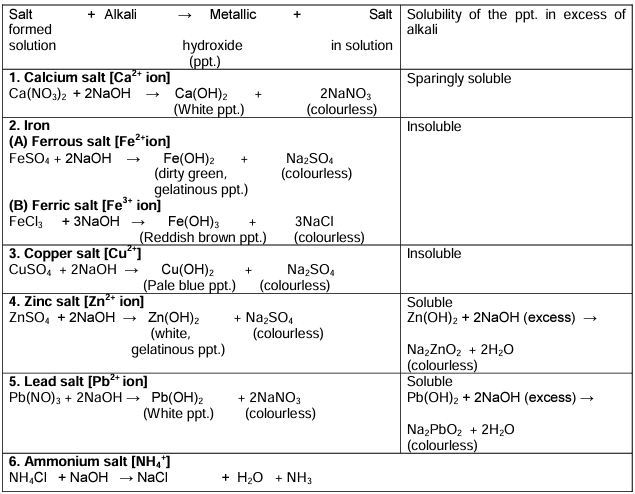
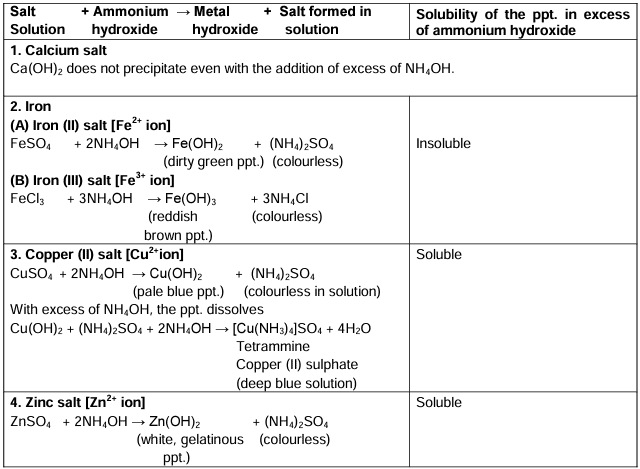
Action of Ammonium Hydroxide on certain Salt Solutions
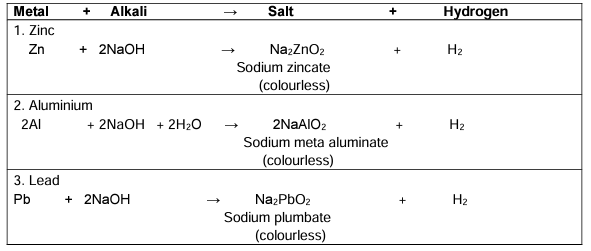
Action of Alkalis on certain Metals
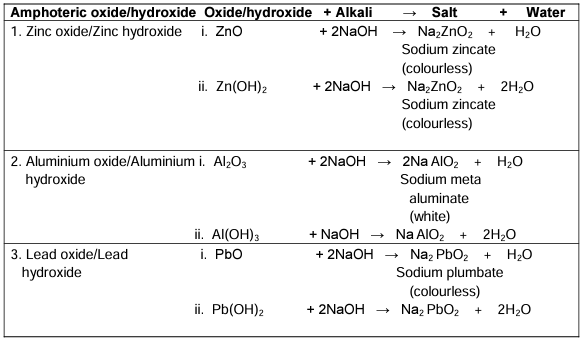
Action of Alkalis on Metal Oxides
Amphoteric oxides and hydroxides: Compounds which react with both acids and alkalis to form salt and water are called amphoteric oxides and hydroxides.
The document Revision Notes: Analytical Chemistry : Uses of Ammonium Hydroxide and Sodium Hydroxide | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
40 videos|140 docs|14 tests
|
FAQs on Revision Notes: Analytical Chemistry : Uses of Ammonium Hydroxide and Sodium Hydroxide - Chemistry Class 10 ICSE
| 1. What are the common uses of ammonium hydroxide in analytical chemistry? |  |
Ans. Ammonium hydroxide is commonly used as a reagent in analytical chemistry for the following purposes: it acts as a source of ammonia for complexation reactions, often used in the preparation of ammonium salts, and serves as a buffering agent. Additionally, it is employed in the titration of weak acids and in the analysis of metal ions by precipitating them as hydroxides.
| 2. How does sodium hydroxide interact with metallic salt solutions? |  |
Ans. Sodium hydroxide reacts with metallic salt solutions to form precipitates of metal hydroxides. For example, when sodium hydroxide is added to copper(II) sulfate solution, a blue precipitate of copper(II) hydroxide is formed. This reaction is useful in identifying metal ions based on the color and solubility of the resulting hydroxide precipitate.
| 3. What is the effect of ammonium hydroxide on certain salt solutions? |  |
Ans. Ammonium hydroxide can cause the precipitation of metal hydroxides from salt solutions. For instance, when added to iron(III) chloride, it produces a reddish-brown precipitate of iron(III) hydroxide. This reaction is significant for qualitative analysis in determining the presence of specific metal ions in a solution.
| 4. How do alkalis like sodium hydroxide and ammonium hydroxide react with metals? |  |
Ans. Alkalis such as sodium hydroxide and ammonium hydroxide react with certain metals to produce hydrogen gas and metal hydroxides. For example, when sodium hydroxide reacts with aluminum, it generates hydrogen gas and sodium aluminate. This property is utilized in various applications, including the extraction of metals from their ores.
| 5. What is the action of alkalis on metal oxides in analytical chemistry? |  |
Ans. Alkalis react with metal oxides to form metal hydroxides. For example, when sodium hydroxide is added to zinc oxide, it reacts to produce sodium zincate and water. This reaction is important for the solubility of metal oxides in alkaline solutions and is used in the preparation and characterization of various metal compounds.
Related Searches
















