Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Revision Notes: Mole Concept and Stoichiometry
Revision Notes: Mole Concept and Stoichiometry | Chemistry Class 10 ICSE PDF Download
Gas Laws
Boyle’s Law
The volume of a given mass of a dry gas is inversely proportional to its pressure at a constant temperature. P1V1 = P2V2 = k at constant temperature
Charles’s Law
The volume of a given mass of a dry gas is directly proportional to its absolute temperature if the pressure is kept constant.
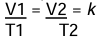 at constant pressure.
at constant pressure.Gas Equation
The volume of a given mass of a dry gas is inversely proportional to the pressure and directly proportional to the absolute temperature.
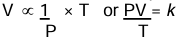
Standard or Normal Temperature and Pressure
- For temperature: 0°C or 273 K
- For pressure: 760 mm or 76 cm of Hg
Gay-Lussac’s law of combining volumes
At the same temperature and pressure, the volume of gases taking part in a chemical reaction as either reactants or products bears a whole number ratio to one another.
Example:
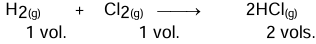
The ratio of reacting gases and products is 1:1:2, which is a simple ratio.
Avogadro’s Law
Under the same conditions of temperature and pressure, equal volumes of all the gases contain the same number of molecules.
Example: A molecule of NH3 is made of one atom of nitrogen and three atoms of hydrogen.
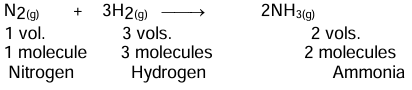
Atomicity
The number of atoms in a molecule of an element is called its atomicity.
- Monatomic: It is composed of only one atom. Examples: Inert gases such as Helium, Neon etc.
- Diatomic: It is composed of two identical atoms. Examples: H2, O2, Cl2 etc.
- Triatomic: It is composed of three identical atoms. Example: Ozone (O3)
- Tetratomic: It is composed of four identical atoms. Example: Phosphorus (P4)
- Octatomic: It is composed of eight identical atoms. Example: Sulphur (S8)
Atomic Mass or Relative Atomic Mass
It is the number which represents how many times one atom of an element is heavier than 1/12th the mass of an atom of carbon-12 (12C).
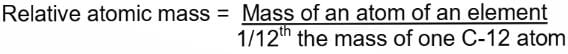
Molecular Mass or Relative Molecular Mass
It is the number which represents how many times one molecule of an element is heavier than 1/12th the mass of an atom of carbon-12 (12C).
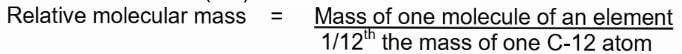
Gram Atomic Mass
The atomic mass of an element expressed in gram is called gram atomic mass.
Example: Gram atomic mass of oxygen is 16 gram.
Gram Molecular Mass
The molecular mass of a substance expressed in gram is called gram molecular mass or molar mass.
Example: Gram molecular mass of water is 18 gram.
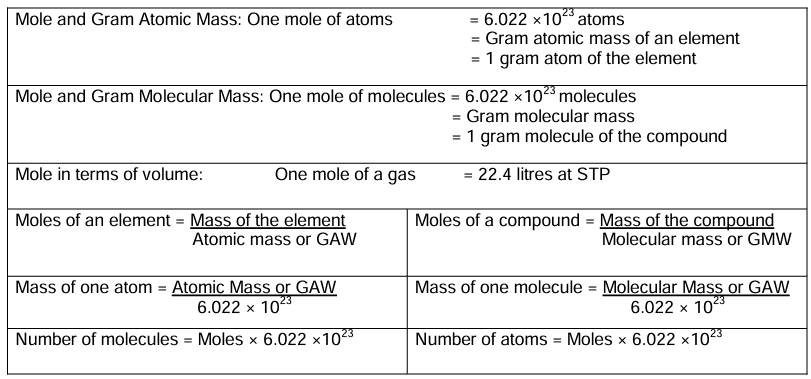
Mole Concept
A mole is a collection of 6.022 × 1023 particles.
A mole is defined as the amount of a substance containing elementary particles such as atoms, molecules or ions in 12 gram of carbon-12 (12C).
Avogadro’s Number
It is defined as the number of atoms present in 12 gram of C-12 isotope, i.e. 6·023 × 1023 atoms. It is denoted by NA or L.
NA = 6·023 × 1023
1 mole of atoms = 6·023 × 1023 atoms
1 mole of molecules = 6·023 × 1023 molecules
1 mole of electrons = 6·023 × 1023 electrons
1 mole of a gas = 22·4 litre at STP
Applications of Avogadro’s Law
- It explains Gay-Lussac’s law.
- It determines atomicity of the gases.
- It determines the molecular formula of a gas.
- It determines the relation between molecular mass and vapour density.
- It gives the relationship between gram molecular mass and gram molar volume.
Relative Vapour Density (VD)
Relative vapour density is the ratio between the masses of equal volumes of a gas (or vapour) and hydrogen under the same conditions of temperature and pressure.
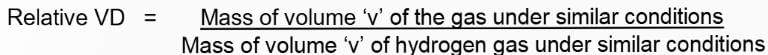 Relative molecular mass of a gas or vapour = 2 × VD
Relative molecular mass of a gas or vapour = 2 × VD
Important Formulae
Percentage Composition
The percentage by weight of each element present in a compound is called percentage composition of the compound.
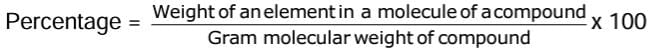
Empirical Formula
It is the chemical formula which gives the simplest ratio in whole numbers of atoms of different elements present in one molecule of the compound.
Empirical Formula Mass
It is the sum of atomic masses of various elements present in the empirical formula.
Empirical Formula Weight (EFW)
The empirical formula weight is the atomic masses of the elements present in the empirical formula.
EFW of H2O2 = 2 × (H) + 2 × (0)
= 2 × 1 + 2 × 16
= 34 amu
Molecular Formula
It denotes the actual number of atoms of different elements present in one molecule of the compound.
Molecular formula = Empirical formula × n
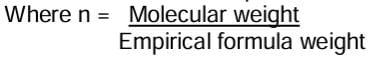
Relationship between Empirical Formula and Molecular Formula
Molecular formula = Empirical formula × n
Where ‘n’ is a positive whole number

Chemical Equation
A shorthand notation of describing an actual chemical reaction in terms of symbols and formula along with the number of atoms and molecules of the reactants and products is called a chemical equation.
A chemical equation is a balanced account of a chemical transaction.
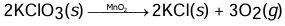
The document Revision Notes: Mole Concept and Stoichiometry | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
39 videos|85 docs|14 tests
|
FAQs on Revision Notes: Mole Concept and Stoichiometry - Chemistry Class 10 ICSE
| 1. What is the mole concept and why is it important in chemistry? |  |
Ans. The mole concept is a fundamental principle in chemistry that relates the amount of substance to its mass, atomic mass, or molecular mass. One mole of any substance contains approximately \(6.022 \times 10^{23}\) particles (atoms, molecules, or ions), known as Avogadro's number. This concept is important because it allows chemists to convert between mass and number of particles, facilitating stoichiometric calculations in chemical reactions.
| 2. How do you calculate the molar mass of a compound? |  |
Ans. To calculate the molar mass of a compound, you add up the atomic masses of each element in the compound, multiplied by the number of times each element appears in the formula. For example, to find the molar mass of water (H₂O), you would calculate: \(2 \times 1.01 \, \text{g/mol (H)} + 16.00 \, \text{g/mol (O)} = 18.02 \, \text{g/mol}\).
| 3. What is the relationship between moles and grams in stoichiometry? |  |
Ans. In stoichiometry, the relationship between moles and grams is established through the molar mass of the substance. To convert grams to moles, you divide the mass of the substance by its molar mass. Conversely, to convert moles to grams, you multiply the number of moles by the molar mass. This relationship is essential for balancing chemical equations and performing quantitative analysis in reactions.
| 4. What is relative vapor density and how is it calculated? |  |
Ans. Relative vapor density (RVD) is a measure of the density of a gas compared to the density of air (which is taken as 1). It is calculated using the formula: \( \text{RVD} = \frac{\text{Molar mass of gas}}{\text{Molar mass of air}} \). For instance, if the molar mass of a gas is 28 g/mol, the RVD would be \( \frac{28}{29} \approx 0.97 \), indicating that the gas is lighter than air.
| 5. What is the difference between gram atomic mass and gram molecular mass? |  |
Ans. Gram atomic mass is the mass of one mole of an element, expressed in grams per mole, and is numerically equal to the atomic mass of the element in atomic mass units (amu). For example, the gram atomic mass of carbon is approximately 12 g/mol. Gram molecular mass, on the other hand, is the mass of one mole of a molecular compound and is the sum of the gram atomic masses of all atoms in the molecule. For instance, the gram molecular mass of CO₂ (carbon dioxide) is \(12 + 2 \times 16 = 44 \, \text{g/mol}\).
Related Searches





















