Revision Notes: Electrolytes Non-Electrolytes and Electrolysis | Chemistry for EmSAT Achieve PDF Download
Electrolysis
Electrolysis
It is the process of decomposition of a chemical compound in aqueous solution or in a molten state, accompanied by a chemical change using direct current.
Electrolyte
It is an ionic compound which in the fused state or in the aqueous solution allows the passage of an electric current and is decomposed by it.
Strong Electrolytes
The compounds which in their aqueous solution or in the fused state are almost completely ionised are called strong electrolytes.
Examples: Mineral acids, alkalis and salts
Weak Electrolytes
The compounds which in their fused state or aqueous solution are feebly ionised and are poor conductors of electricity are called weak electrolytes.
Examples: Acetic acid, oxalic acid
Non-electrolytes
The compounds which neither in solution nor in the molten state allow an electric current to pass through them are called non-electrolytes.
Examples: Kerosene, carbon disulphide
Electrolytic Cell
It is a device used to convert electrical energy into chemical energy.
Electrochemical Cell
It is a device used to convert chemical energy into electrical energy.
Electrodes
The graphite, metal plates or gas carbon rods immersed in the electrolyte through which current enters and leaves the electrolytic cell are called electrodes.
Cathode
The electrode connected to the negative terminal of the battery is called a cathode.
Anode
The electrode connected to the positive terminal of the battery is called an anode.
Ions
The atoms or groups of atoms which carry a positive or negative charge are known as ions.
Cations
Atoms which carry a positive charge are called cations.
Anions
Atoms which carry a negative charge are called anions.
Oxidation
It is a chemical process which involves the addition of oxygen or the removal of hydrogen.
Oxidising Agents
It is a substance which oxidises other substances either by accepting electrons or by providing oxygen or an electronegative ion.
Reduction
It is a chemical process which involves the removal of oxygen or the addition of hydrogen.
Reducing Agent
It is a substance which reduces other substances by providing electrons, hydrogen or an electropositive ion.
Dissociation
The process due to which an ionic compound dissociates into ions in the fused state or in the aqueous solution is called electrolytic dissociation.
Example: Electrovalent compound such as NaCl.
NaCl → Na+ + Cl–
Ionisation
The process by which polar covalent compounds are converted into ions in water solution is called ionisation.
HCl ⇌ H+ + Cl–
Electrochemical Series
It is a series in which metals are arranged based on the ease with which atoms of metals lose electrons to form positively charged ions.
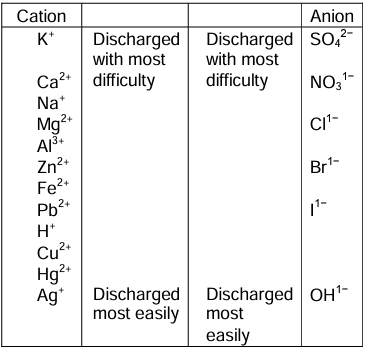
Selective Discharge of Ions
The preferential discharge of ions present in an electrolyte at the respective electrodes is known as selective discharge of ions.
It depends on the following factors:
i. Relative position of ions in an electrochemical series
ii. Concentration of the ions
iii. Nature of the electrode
Electrolysis of Fused Lead Bromide
Electrolyte: Molten lead bromide (PbBr2)
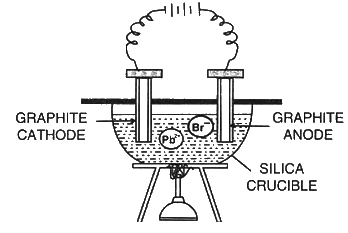
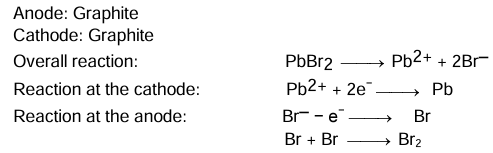
Electrolysis of Acidified Water
Electrolyte: Acidified water
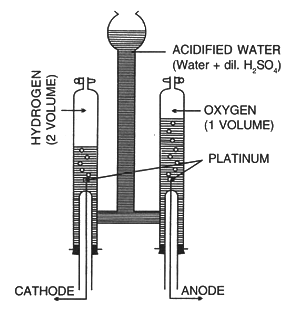
Anode: Platinum
Cathode: Platinum
Ionisation of acidified water:
H2O ⇌ H+ + OH–
H2SO4 ⇌ 2H+ + SO42–
Ions present:
H+ , SO42–, OH–
Reaction at the cathode:
H+ + e− → H
H + H → H2 (Hydrogen molecule)
Reaction at the anode:
OH– − e− → OH × 4
OH– ion discharge in preference to SO42–
4OH → 2H2O + O2 (Oxygen molecule)
Electrolysis of Aqueous Copper Sulphate
Electrolyte: Aqueous copper sulphate solution
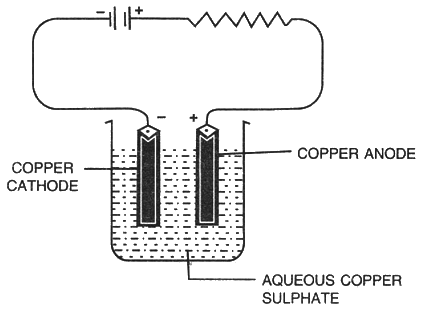
Anode: Copper
Cathode: Copper
Dissociation of aqueous copper sulphate:
CuSO4 ⇌ Cu2+ + SO42–
H2O ⇌ H+ + OH–
Reaction at the cathode: Cu2+ + 2e− → Cu
Cu being lower in the electrochemical series.
Reaction at the anode: Cu - 2e- → Cu2+
SO42– and OH– are not discharged.
Applications of Electrolysis
i. Electroplating with metals
ii. Electrorefining of metals
iii. Extraction of metals
Electroplating
It is a process in which a thin film of a metal, such as gold, silver or nickel, gets deposited on another metallic article with the help of electricity.
Reasons for Electroplating
i. Decoration purposes
ii. To protect from rusting and corrosion
Electroplating with Nickel
Electrolyte: Aqueous solution of nickel sulphate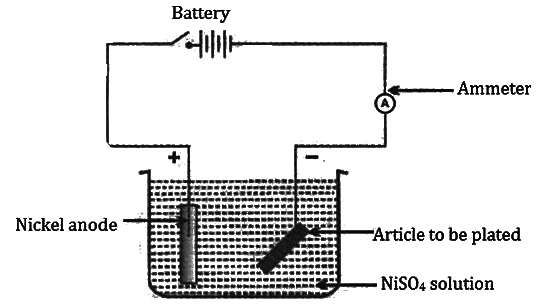
Dissociation:
NiSO4 ⇌ Ni2+ + SO42−
H2O ⇌ H+ + OH−
Cathode: Article to be electroplated
Anode: Block of pure nickel
Reaction at cathode: Ni2+ 2e- → Ni (deposited)
Reaction at anode: Ni − 2e- → Ni2+
Note: Article to be electroplated is always kept at the cathode.
Electrolytic refining of metals
It is a process by which metals containing impurities are purified electrolytically to give a pure metal.
Electrolytic refining of copper
Electrolyte: Copper sulphate solution and dil. sulphuric acid
Cathode: Thin strip of pure copper
Anode: Impure copper
Reaction at cathode: Cu2+ + 2e- → Cu
Reaction at anode: Cu − 2e- → Cu2+
Electrometallurgy
It is the process of extraction of metals by electrolysis.
Activity Series
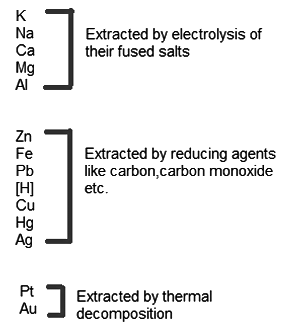
Acids, Bases and Salts as Electrolytes
They can be classified as strong or weak electrolytes depending on the degree of dissociation.
Degree of dissociation = (Number of molecules dissociated/Total number of molecules) × 100
|
191 videos|265 docs|160 tests
|
FAQs on Revision Notes: Electrolytes Non-Electrolytes and Electrolysis - Chemistry for EmSAT Achieve
| 1. What is electrolysis and how does it work? |  |
| 2. What are the main applications of electrolysis? |  |
| 3. Why is electroplating used and what materials are commonly used for electroplating? |  |
| 4. How does electrolytic refining work, specifically for copper? |  |
| 5. What is the role of acids, bases, and salts as electrolytes in electrolysis? |  |
















