Revision Notes: Study of Compounds - Hydrogen Chloride | Chemistry Class 10 ICSE PDF Download
Study of Compounds – Hydrogen Chloride
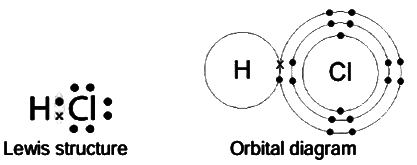
Hydrogen Chloride
Molecular formula: HCl
Molecular mass: 36.5 amu
Bond: Covalent
General Preparation of HCl gas
i. By synthesis
Moist hydrogen gas combines with chlorine in the presence of diffused sunlight.
ii. By heating metallic chloride with conc. sulphuric acid
Laboratory Preparation of Hydrogen Chloride
Hydrogen chloride gas is prepared by heating a metallic chloride (NaCl) with conc. sulphuric acid (H2SO4).
Reactions: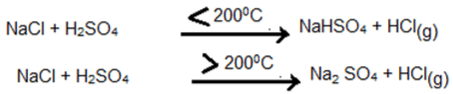
Collection
- Hydrogen chloride gas is collected by the upward displacement of air as it is 1.28 times heavier than air.
- It is not collected over water because it is highly soluble in water.
Physical Properties
- Colourless, pungent, choking odour, slight sour taste.
- It is 1.28 times heavier than water and highly soluble in water.
- Liquefies at temperature of about 10°C at 40 atmospheric pressure.
- Boiling point is −83°C, and freezing point is −113°C.
Chemical Properties of HCl
- Combustibility: The gas is neither combustible nor a supporter of combustion.
- Thermal dissociation: On heating above 500°C, it dissociates into hydrogen and chlorine.
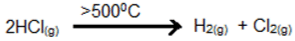
- With metals: Metals which come before hydrogen in the electrochemical series form chlorides with the liberation of hydrogen.
Zn + 2HCl → ZnCl2 + H2(g) - Reaction with ammonia: It combines with ammonia to form dense white fumes of ammonium chloride.
NH3 (g) + HCl (g) → NH4Cl
Hydrochloric Acid
Hydrochloric acid is prepared by dissolving hydrogen chloride gas in water using a special funnel arrangement because direct absorption of HCl gas in water using a delivery tube causes back suction.Properties of Hydrochloric Acid
Physical Properties
- Colourless, slightly pungent with sharp sour taste.
- Corrosive in nature and causes blisters on the skin.
- Density is 1.2 gm/cc with boiling point of 110°C.
Chemical Properties
- Monobasic in nature
HCl dissociates in aqueous solution to produce one hydrogen ion [H+] per molecule of the acid.
HCl + H2O → H3O+ + Cl- - Acidic nature
The presence of hydrogen ion [H+] in HCl imparts acidic properties to an aqueous solution of hydrochloric acid. - Action on metals
Ca + 2HCl → CaCl2 + H2
Mg + 2HCl → MgCl2 + H2 - Action on oxides and hydroxides
MgO + 2HCl → MgCl2 + H2O
Ca(OH)2 + 2HCl → CaCl2 + H2O - With salts of weaker acids
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
NaHCO3 + HCl → NaCl + H2O + CO2
Na2SO3 + 2HCl → 2NaCl + H2O + CO2
NaHSO3 + HCl → NaCl + H2O + SO2
Na2S + 2HCl → 2NaCl + H2S - Action on thiosulphates
Na2SO3 + 2HCl → 2NaCl + H2O + SO2 + S - Reaction with nitrates
AgNO3 + HCl → AgCl + HNO3
Oxidation of Hydrochloric Acid
- MnO2 + 4HCl
 MnCl2 + 2H2O + Cl2
MnCl2 + 2H2O + Cl2 - K2Cr2O7 + 14HCl
 2KCl + 2CrCl3 + 7H2O + 3Cl2
2KCl + 2CrCl3 + 7H2O + 3Cl2 - 2KMnO4 + 16HCl
 2KCl + 2MnCl2 + 8H2O + 5Cl2
2KCl + 2MnCl2 + 8H2O + 5Cl2 - Pb3O4 + 8HCl
 3 PbCl2 + 4H2O + Cl2
3 PbCl2 + 4H2O + Cl2
Formation of Aqua Regia
Aqua regia is a mixture of one part of conc. nitric acid and three parts of conc. hydrochloric acid.
HNO3 + 3HCl → NOCl + 2H2O + 2[Cl]
The nascent chlorine released reacts with noble metals such as gold and platinum to give their soluble chlorides.
Au + 3[Cl] → AuCl3
Pt + 4[Cl] → PtCl4
Uses of Hydrochloric Acid
a. In the manufacture of dyes, drugs, paints and silver chloride.
b. For purifying bone black, because HCl dissolves the calcium phosphate present in bones.
c. To remove rust from iron sheets.
|
39 videos|85 docs|14 tests
|
FAQs on Revision Notes: Study of Compounds - Hydrogen Chloride - Chemistry Class 10 ICSE
| 1. What is the general method for preparing hydrogen chloride gas in the laboratory? |  |
| 2. How is hydrogen chloride gas collected during its preparation? |  |
| 3. What are the physical properties of hydrogen chloride? |  |
| 4. What are some chemical properties of hydrochloric acid? |  |
| 5. What are the properties of hydrochloric acid that make it useful in various applications? |  |
















