Structure of Atom Revision Notes | Chemistry Class 11 - NEET PDF Download
Introduction
Atoms are fundamental units of matter, once thought indivisible but now known to consist of subatomic particles: electrons, protons, and neutrons. Understanding atomic structure is key to explaining chemical and physical properties of elements.
Discovery of Subatomic Particles
1. Electron (J.J. Thomson, 1897):
- Identified via cathode ray experiments; negatively charged, mass ~1/1837 of H atom.
- Charge: -1.602 × 10⁻¹⁹ C; Mass: 9.109 × 10⁻³¹ kg.
2. Proton (Rutherford, 1919):
- Found via anode rays (canal rays); positively charged, mass ~1836 times electron.
- Charge: +1.602 × 10⁻¹⁹ C; Mass: 1.672 × 10⁻²⁷ kg.
3. Neutron (James Chadwick, 1932):
- Neutral particle, slightly heavier than proton; Mass: 1.675 × 10⁻²⁷ kg.
- Charge and Mass Comparison:
Atomic Models
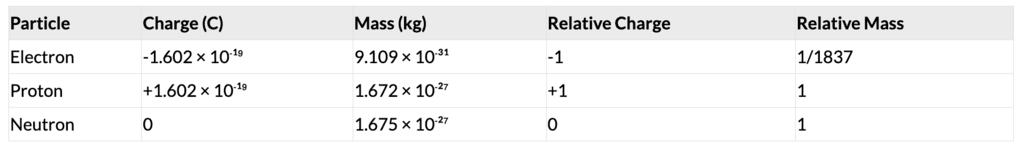
1. Thomson’s Model (1904)
- Atom as a positively charged sphere with embedded electrons (plum pudding model).
- Limitation: Could not explain electron stability or spectral lines.
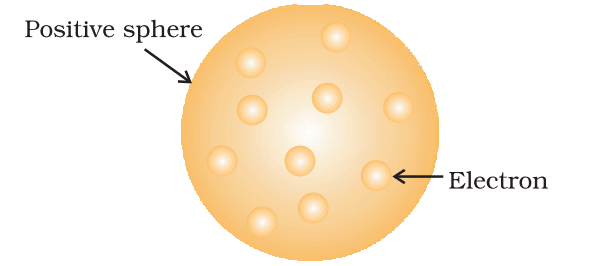
2. Rutherford’s Model (1911)
- Based on alpha particle scattering:
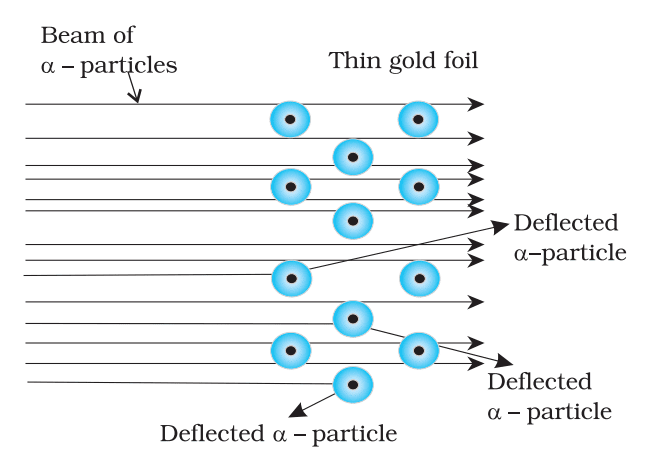
- Nucleus: Small, dense, positively charged center containing most mass.
- Electrons: Orbit nucleus like planets around the sun.
- Limitation: Electrons should emit energy and spiral into nucleus, contradicting atomic stability.
Atomic Number and Mass Number
- Atomic Number (Z): Number of protons (defines element), e.g., H (Z=1), C (Z=6).
- Mass Number (A): Protons + neutrons, e.g., ¹²C (A=12, Z=6, neutrons=6).
- Isotopes: Same Z, different A (e.g., ¹²C, ¹⁴C).
- Isobars: Same A, different Z (e.g., ¹⁴C, ¹⁴N).
- Notation: ⁿₘX, where X is element, m is A, n is Z (e.g., ¹²₆C).
Developments Leading to Bohr’s Model
- Electromagnetic Waves: Maxwell’s theory; light as waves with wavelength (λ), frequency (ν), speed (c = λν).
- Planck’s Quantum Theory (1900): Energy emitted/absorbed in quanta (E = hν, h = 6.626 × 10⁻³⁴ J s).
- Einstein’s Photoelectric Effect (1905): Light quanta (photons) eject electrons; energy threshold required.
- Atomic Spectra:
- Emission: Light emitted by energized atoms (e.g., hydrogen’s line spectrum).
- Absorption: Light absorbed by atoms, forming dark lines.
- Hydrogen Spectrum: Lyman (UV), Balmer (visible), Paschen, Brackett, Pfund (IR); Rydberg formula: 1/λ = R(1/n₁² - 1/n₂²), R = 109677 cm⁻¹.
Bohr’s Model for Hydrogen Atom (1913)
- Postulates:
- Electrons orbit nucleus in fixed energy levels (orbits) without radiating energy.
- Energy levels quantized: Eₙ = -2.18 × 10⁻¹⁸/n² J (n = principal quantum number).
- Electron transitions emit/absorb energy: ΔE = E₂ - E₁ = hν.
- Radius: rₙ = 0.529 × n²/Z Å (H: n=1, r=0.529 Å).
- Velocity: vₙ = 2.188 × 10⁶ × Z/n m/s.
- Success: Explained hydrogen spectrum, atomic stability.
- Limitations: Failed for multi-electron atoms, ignored electron spin, contradicted Heisenberg’s uncertainty principle.
Towards Quantum Mechanical Model
Dual Nature of Matter:
- de Broglie (1924): Particles have wave properties; λ = h/mv (h = Planck’s constant, m = mass, v = velocity).
- Example: Electron at 100 V, λ = 1.227/√V nm.
Heisenberg’s Uncertainty Principle (1927): Cannot know exact position and momentum simultaneously; Δx × Δp ≥ h/4π.
Quantum Mechanical Model
- Schrödinger’s Wave Equation: Treats electrons as waves, solved for orbitals (probability regions).
- Orbitals: Defined by quantum numbers:
- Principal (n): Energy level (1, 2, 3, …).
- Azimuthal (l): Subshell (0 to n-1; s, p, d, f).
- Magnetic (mₗ): Orbital orientation (-l to +l).
- Spin (mₛ): Electron spin (+1/2 or -1/2).
- Probability Density: ψ² gives electron likelihood.
Shapes of Atomic Orbitals
- s-Orbital (l=0): Spherical, size increases with n.
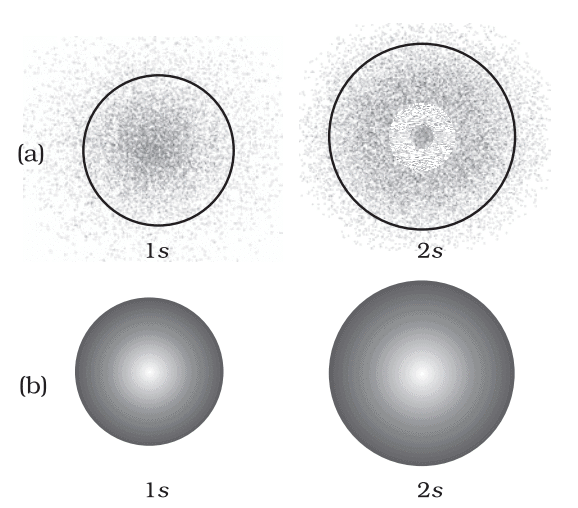
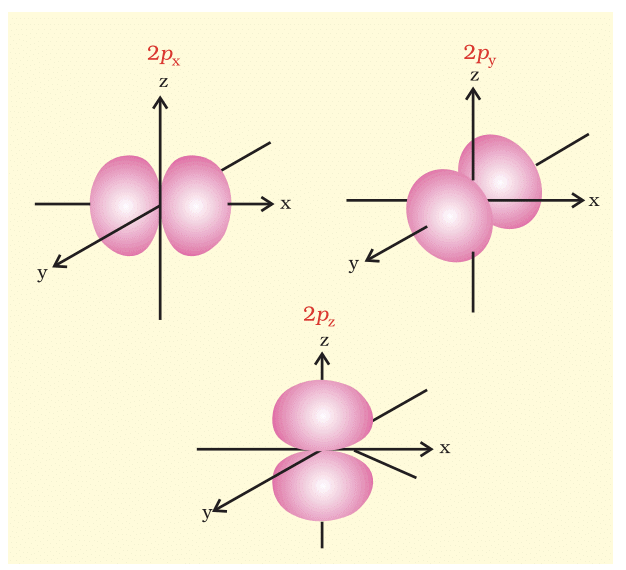
- p-Orbital (l=1): Dumbbell-shaped, three orientations (px, py, pz).
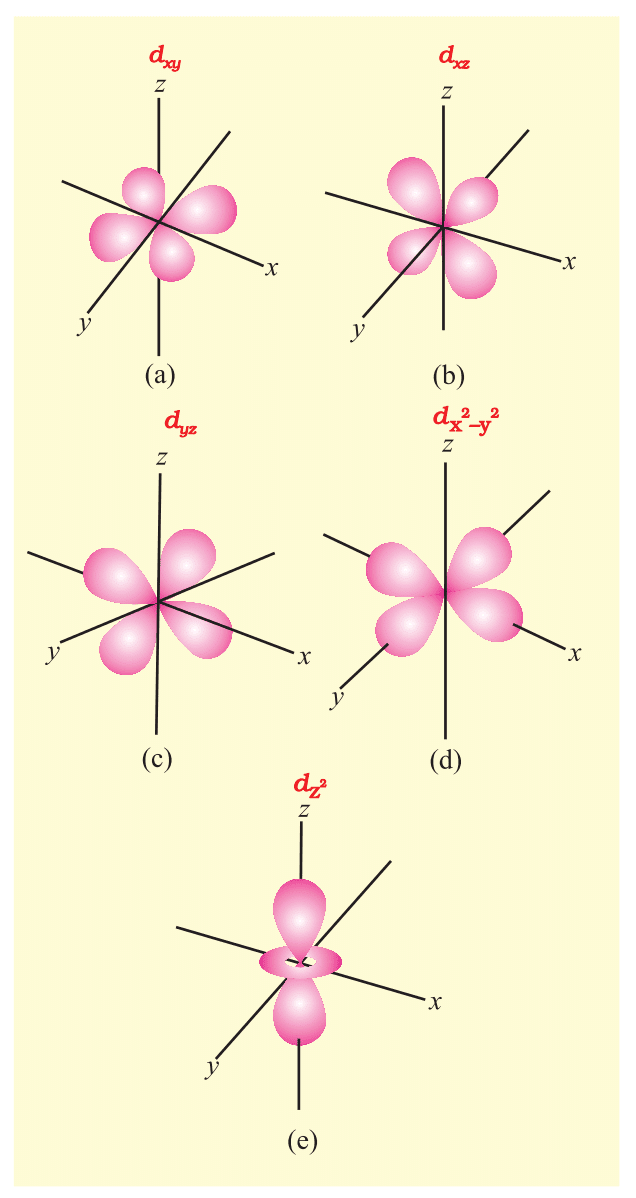
- d-Orbital (l=2): Five orientations, complex shapes.
- Nodes: Regions of zero electron density (e.g., 2s has one spherical node).
Energies of Orbitals
- Hydrogen: Energy depends only on n (Eₙ = -13.6/n² eV).
- Multi-electron Atoms: Energy depends on n and l (e.g., 3s < 3p < 3d due to penetration effect).
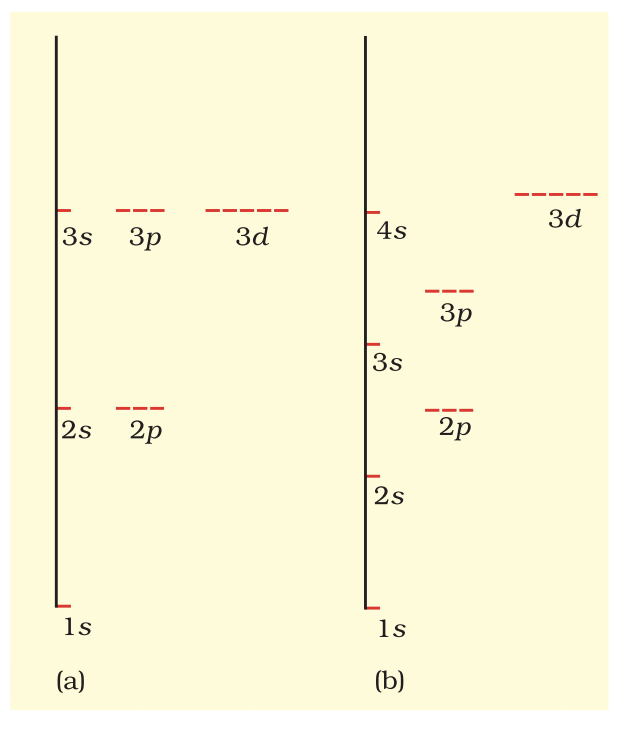 Energy Level Diagram
Energy Level Diagram
Filling of Orbitals
- Aufbau Principle: Electrons fill lowest energy orbitals first (1s, 2s, 2p, 3s, …).
- Pauli Exclusion Principle: No two electrons have identical four quantum numbers; max 2 electrons per orbital with opposite spins.
- Hund’s Rule: Electrons pair only after singly occupying degenerate orbitals with parallel spins.
- Configurations: e.g., C (Z=6): 1s²2s²2p²; exceptions like Cr (3d⁵4s¹) for stability.
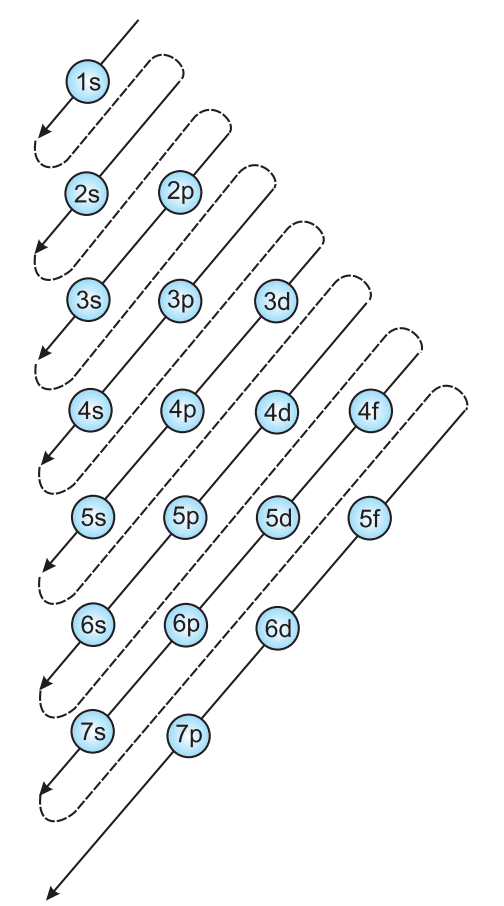 Order of filling of orbitals
Order of filling of orbitals
Summary
Atoms consist of electrons, protons, and neutrons. Thomson’s plum pudding model evolved into Rutherford’s nuclear model, refined by Bohr’s quantized orbits for hydrogen. Quantum mechanics, with de Broglie’s wave-particle duality and Schrödinger’s orbitals, describes electron probability via quantum numbers (n, l, mₗ, mₛ). Orbital shapes (s, p, d) and energy levels guide electron filling per Aufbau, Pauli, and Hund’s rules, explaining atomic properties.
|
114 videos|263 docs|74 tests
|
FAQs on Structure of Atom Revision Notes - Chemistry Class 11 - NEET
| 1. What are the key differences between the classical and quantum models of the atom? |  |
| 2. How do electromagnetic waves relate to atomic spectra? |  |
| 3. What is the significance of quantum numbers in describing electron configurations? |  |
| 4. What is the concept of wave-particle duality in relation to electrons? |  |
| 5. How does the stability of atomic spectra relate to electron configurations? |  |






















