Revision Notes: Solutions | Chemistry Class 12 - NEET PDF Download
Revision Notes: Solutions Introduction
A solution is a homogeneous mixture of two or more substances. The substance present in the largest quantity is the solvent, and the other components are the solutes. Solutions can exist in various forms: solid, liquid, or gaseous mixtures.
Types of Solutions
Solutions can be classified based on the phases of the solute and solvent. The common types are: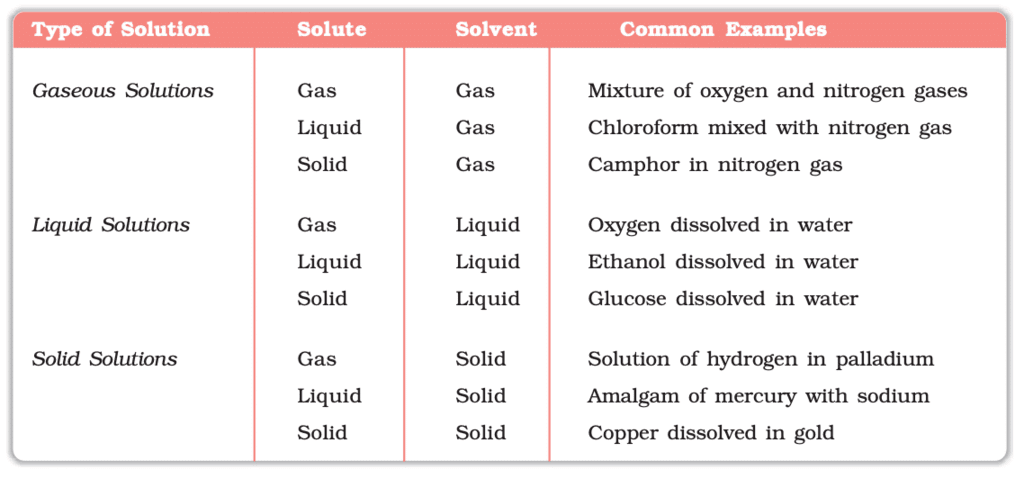
Concentration of Solutions
The concentration of a solution can be expressed in various ways:
- Mass Percentage (w/w): Mass % of a component = (Mass of the component / Total mass of the solution) × 100
- Volume Percentage (V/V): Volume % of a component = (Volume of the component / Total volume of the solution) × 100
- Molarity (M): Molarity (M) = Moles of solute / Volume of solution in liters
- Molality (m): Molality (m) = Moles of solute / Mass of solvent in kilograms
- Mole Fraction (χ): Mole Fraction (χ) = (Moles of component) / (Total moles of all components)
Henry’s Law
Henry’s law states that at constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the solution:
- C = kP (where C is the concentration of the gas, P is the partial pressure, and k is the Henry’s law constant)
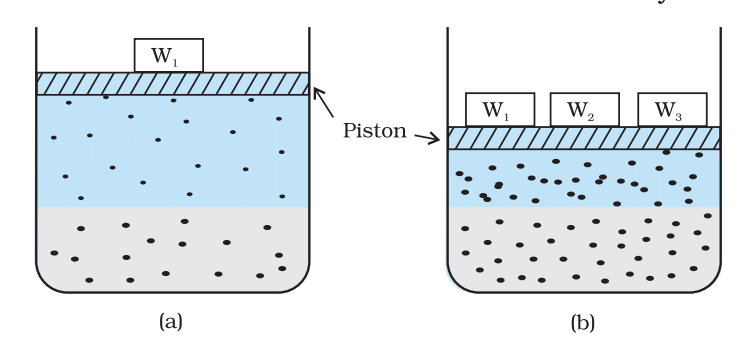 Effect of pressure on the solubility of a gas. Theconcentration of dissolved gas is proportional to thepressure on the gas above the solution
Effect of pressure on the solubility of a gas. Theconcentration of dissolved gas is proportional to thepressure on the gas above the solution
Raoult’s Law
Raoult’s law states that the partial vapor pressure of each volatile component in a solution is directly proportional to its mole fraction in the solution. Mathematically:
p₁ = χ₁ * p₁⁰, where p₁ is the vapor pressure of component 1 in the solution, and p₁⁰ is the vapor pressure of the pure solvent.
Ideal and Non-Ideal Solutions
Solutions can be categorized as:
- Ideal Solutions: Solutions that obey Raoult's law over the entire range of concentration.
- Non-Ideal Solutions: Solutions that do not obey Raoult’s law. They exhibit either positive or negative deviations from Raoult’s law.
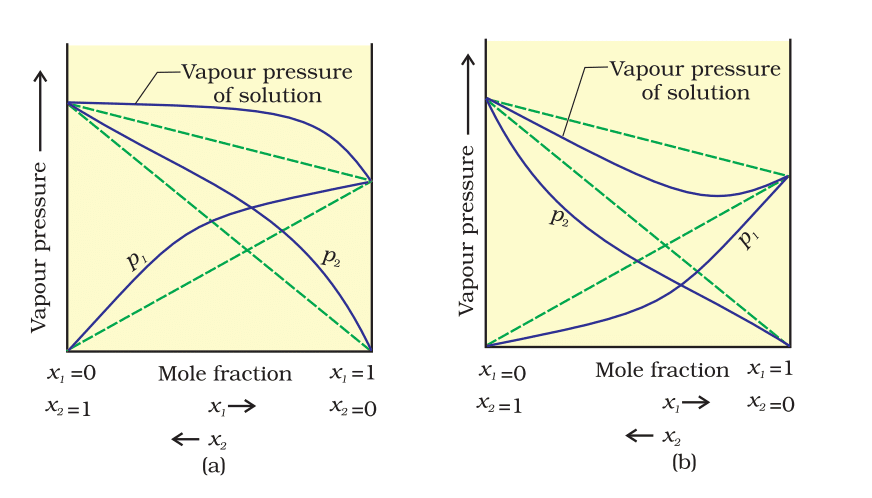 The vapour pressures of two component systems as a function ofcomposition (a) asolution that shows positive deviation from Raoult's law and (b) a solution that shows negative deviation from Raoult's law.
The vapour pressures of two component systems as a function ofcomposition (a) asolution that shows positive deviation from Raoult's law and (b) a solution that shows negative deviation from Raoult's law.
Colligative Properties
Colligative properties depend on the number of solute particles, not on their chemical identity. These properties include:
- Lowering of Vapor Pressure: The vapor pressure of the solvent decreases when a non-volatile solute is added.
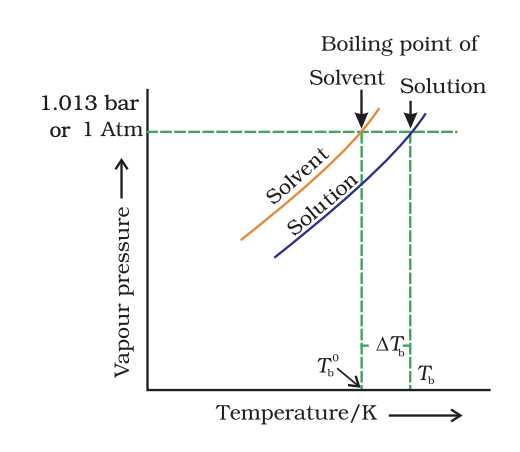 The vapour pressure curve forsolution lies below the curve for purewater. The diagram shows that Tb denotes the elevation of boiling point of a solvent in solution.
The vapour pressure curve forsolution lies below the curve for purewater. The diagram shows that Tb denotes the elevation of boiling point of a solvent in solution. - Elevation of Boiling Point: The boiling point of the solution is higher than that of the pure solvent.
- Depression of Freezing Point: The freezing point of the solution is lower than that of the pure solvent.
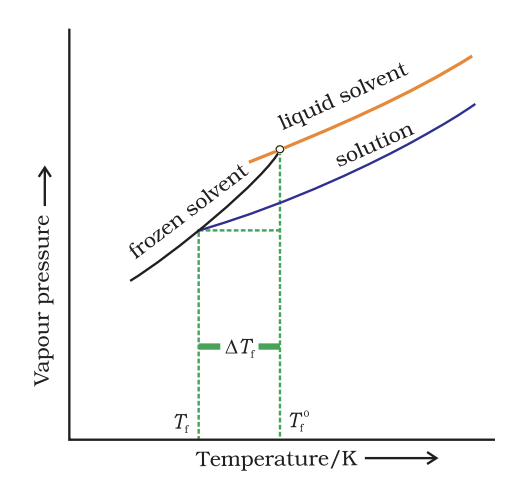 Diagram showing Tf, depressionof the freezing point of a solvent ina solution.
Diagram showing Tf, depressionof the freezing point of a solvent ina solution. - Osmotic Pressure: The pressure required to stop osmosis, which is proportional to the molarity of the solution. The formula is:
π = C × R × T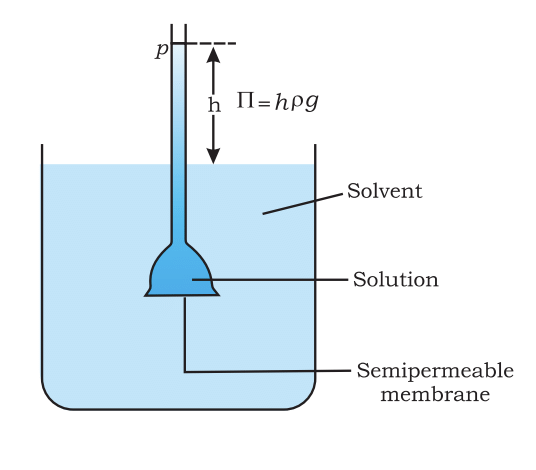 Level of solutionrises in the thistlefunnel due toosmosis of solvent.
Level of solutionrises in the thistlefunnel due toosmosis of solvent.
Examples of Colligative Properties
- Freezing point depression of water when salt is added.
- Boiling point elevation of water when sugar is dissolved.
- Osmotic pressure calculation to find the molar mass of solutes.
Summary
A solution is a homogeneous mixture. Solutions are classified based on the phases of solute and solvent. Concentration can be measured in various units like mass percentage, volume percentage, molarity, molality, and mole fraction. Raoult’s law relates the vapor pressure of a solution to its mole fraction. Colligative properties depend on the number of solute particles and are important for determining properties like boiling point elevation, freezing point depression, and osmotic pressure.
|
75 videos|338 docs|78 tests
|
FAQs on Revision Notes: Solutions - Chemistry Class 12 - NEET
| 1. What are the different types of solutions in chemistry? |  |
| 2. How does temperature affect the solubility of solids and gases? |  |
| 3. What is the difference between molarity and molality? |  |
| 4. What are colligative properties, and why are they important? |  |
| 5. How can you prepare a dilute solution from a concentrated solution? |  |

















