NEET Exam > NEET Notes > Chemistry Class 11 > Revision Notes: Redox Reactions
Revision Notes: Redox Reactions | Chemistry Class 11 - NEET PDF Download
Introduction
Redox reactions involve simultaneous oxidation and reduction processes, playing a key role in chemistry. They are essential in physical and biological phenomena, with applications in pharmaceuticals, industry, metallurgy, agriculture, and environmental issues like the Hydrogen Economy and Ozone Hole.
Classical Idea of Redox Reactions
- Oxidation: Originally, addition of oxygen (e.g., 2Mg(s) + O2(g) → 2MgO(s)), later expanded to removal of hydrogen (e.g., 2H2S(g) + O2(g) → 2S(s) + 2H2O(l)) or electropositive elements.
- Reduction: Initially, removal of oxygen (e.g., 2HgO(s) → 2Hg(l) + O2(g)), later included addition of hydrogen (e.g., CH2=CH2(g) + H2(g) → H3C-CH3(g)) or electropositive elements.
- Key Insight: Oxidation and reduction occur together, forming "redox" reactions.
Electron Transfer Perspective
- Oxidation: Loss of electrons (e.g., 2Na(s) → 2Na+(g) + 2e-).
- Reduction: Gain of electrons (e.g., Cl2(g) + 2e- → 2Cl-(g)).
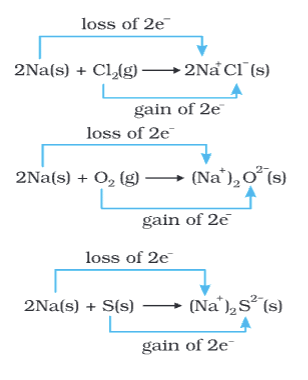
- Half Reactions: Redox splits into oxidation and reduction halves (e.g., 2Na(s) + Cl2(g) → 2NaCl(s)).
- Agents: Oxidizing agent accepts electrons (e.g., Cl2), reducing agent donates electrons (e.g., Na).
- Competitive Reactions: Zn reduces Cu2+ (Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)), Cu reduces Ag+, showing order Zn > Cu > Ag.
Oxidation Number
- Definition: Charge assigned assuming electron transfer to the more electronegative atom (e.g., H2O: H = +1, O = -2).
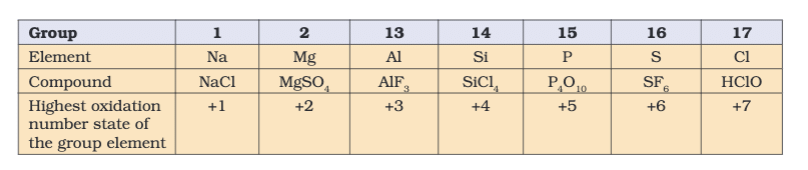
- Rules:
- Elements in free state: 0 (e.g., H2, O2).
- Monoatomic ions: Charge equals oxidation number (e.g., Na+ = +1).
- Oxygen: -2 (except -1 in peroxides like H2O2, +2 in OF2).
- Hydrogen: +1 (except -1 in metal hydrides like NaH).
- Fluorine: -1 always.
- Sum in compounds = 0, in ions = charge (e.g., CO32- = -2).
- Stock Notation: Roman numerals for oxidation state (e.g., Fe(II)O, Fe(III)2O3).
- Redox Identification: Increase in oxidation number = oxidation, decrease = reduction (e.g., 2Cu2O(s) + Cu2S(s) → 6Cu(s) + SO2(g)).
Types of Redox Reactions
- Combination: A + B → C, elements combine (e.g., C(s) + O2(g) → CO2(g)).
- Decomposition: AB → A + B, compound breaks (e.g., 2H2O(l) → 2H2(g) + O2(g)).
- Displacement: X + YZ → XZ + Y (e.g., Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)).
- Disproportionation: Element oxidizes and reduces (e.g., 2Cu+(aq) → Cu(s) + Cu2+(aq)).
Balancing Redox Reactions
- Oxidation Number Method:
- Write formulas, assign oxidation numbers.
- Equalize oxidation number changes.
- Add H+/OH- and H2O to balance charges and atoms.
- Example: Cr2O72- + 3SO32- + 8H+ → 2Cr3+ + 3SO42- + 4H2O.
- Half Reaction Method:
- Split into oxidation and reduction halves.
- Balance atoms, add H2O/H+, then electrons.
- Equalize electrons, combine halves.
- Example: 6Fe2+ + Cr2O72- + 14H+ → 6Fe3+ + 2Cr3+ + 7H2O.
Redox Titrations
- Self-Indicator: MnO4- turns pink at endpoint.
- External Indicator: Cr2O72- with diphenylamine (blue).
- Iodine Method: Cu2+ + I- → I2, titrated with S2O32-, starch turns blue.
Electrode Processes
- Daniell Cell: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s), electrons flow via wire, ions via salt bridge.
- Redox Couple: Oxidized/reduced forms (e.g., Zn2+/Zn).
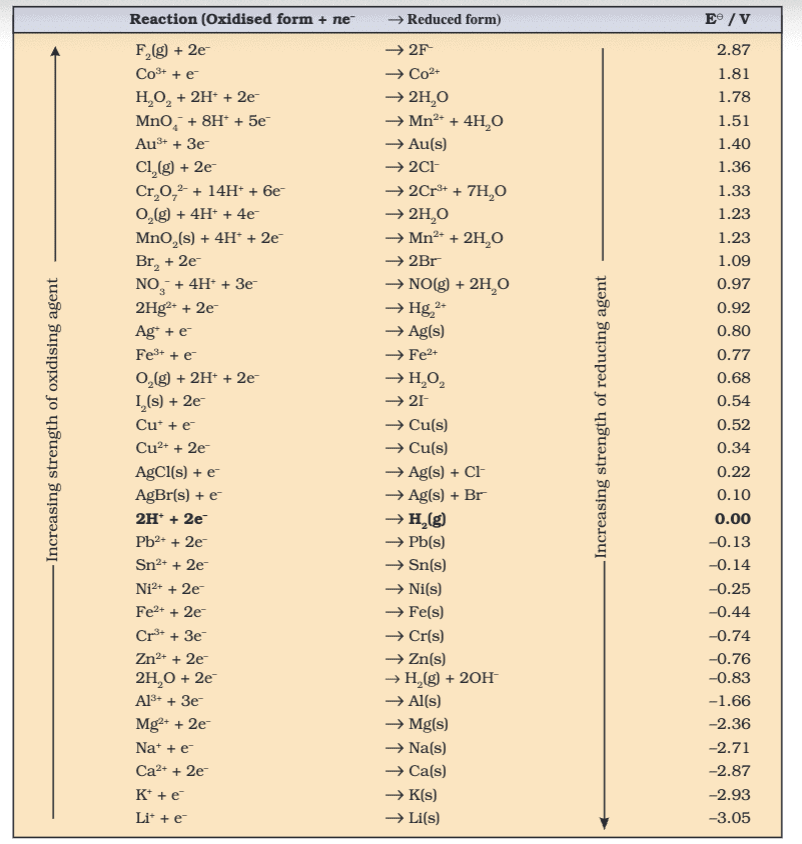
- Electrode Potential: Tendency to gain/lose electrons, standard E° at 298 K, 1 atm, 1 M (e.g., H+/H2 = 0 V).
- Table - Standard Electrode Potentials:
Summary
Redox reactions involve oxidation (electron loss) and reduction (electron gain), identified via classical (O/H changes), electron transfer, or oxidation number methods. They are classified as combination, decomposition, displacement, or disproportionation. Balancing uses oxidation number or half-reaction methods. Applications include titrations and electrochemical cells (e.g., Daniell cell), with electrode potentials indicating reactivity.
The document Revision Notes: Redox Reactions | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Revision Notes: Redox Reactions - Chemistry Class 11 - NEET
| 1. What are redox reactions and how do they occur? |  |
Ans. Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons between two species. In these reactions, one substance gets oxidized (loses electrons) while another gets reduced (gains electrons). The oxidation state of the substances changes, facilitating energy transfer. Common examples include combustion, respiration, and photosynthesis.
| 2. How do you identify the oxidizing and reducing agents in a redox reaction? |  |
Ans. To identify the oxidizing and reducing agents, first assign oxidation states to all elements in the reaction. The substance that undergoes oxidation (increases in oxidation state) is the reducing agent, while the substance that undergoes reduction (decreases in oxidation state) is the oxidizing agent. For example, in the reaction between zinc and copper sulfate, zinc is oxidized and acts as the reducing agent, whereas copper(II) ions are reduced and act as the oxidizing agent.
| 3. What is the significance of redox reactions in biological systems? |  |
Ans. Redox reactions are crucial in biological systems as they play a key role in metabolic processes, such as cellular respiration and photosynthesis. In cellular respiration, glucose is oxidized to produce energy, while oxygen is reduced to form water. Similarly, in photosynthesis, carbon dioxide is reduced to form glucose, while water is oxidized to release oxygen. These reactions are essential for energy transfer and the maintenance of life.
| 4. How can redox reactions be balanced in chemical equations? |  |
Ans. Balancing redox reactions can be done using the half-reaction method. First, separate the oxidation and reduction reactions. Then, balance the atoms and charges in each half-reaction. Finally, combine the half-reactions to form a balanced overall equation, ensuring that the electrons lost in oxidation equal the electrons gained in reduction. This method is effective for both acidic and basic solutions by adjusting for H+ and OH- ions as needed.
| 5. What are common examples of redox reactions in everyday life? |  |
Ans. Common examples of redox reactions include rusting of iron (oxidation of iron in the presence of moisture), combustion of fuels (like gasoline burning in engines), and the reactions in batteries (where chemical energy is converted into electrical energy). These reactions illustrate the principles of electron transfer and energy conversion in real-world applications.
Related Searches
















