Revision Notes: Some Basic Concepts of Chemistry | Chemistry Class 11 - NEET PDF Download
Introduction
Chemistry studies molecules and their transformations, focusing on the infinite variety of molecular combinations from about 100 elements. It systematizes knowledge to describe and understand nature, dealing with diverse substances and their changes in daily life (e.g., curd formation, rusting).
Development of Chemistry
- Ancient India: Known as Rasayan Shastra, included metallurgy, medicine, and production of cosmetics, glass, dyes. Archaeological evidence (e.g., Mohenjodaro, Harappa) shows early chemical processes like pottery and metal forging.
- Key Contributions: Nagarjuna (chemist, and alchemist) wrote Rasratnakar on mercury compounds; Chakrapani discovered mercury sulphide and invented soap.
- Historical Context: Alchemy and Iatrochemistry (1300-1600 CE) evolved into modern chemistry in 18th-century Europe, influenced by Arab traditions.
Importance of Chemistry
- Applications: Weather patterns, brain function, chemical industries (fertilizers, drugs, polymers), and new materials (superconducting ceramics, optical fibres).
- Impact: Enhances national economy, meets human needs (food, healthcare), and addresses environmental issues (e.g., safer refrigerants).
Nature of Matter
- Definition: Anything with mass and occupying space.
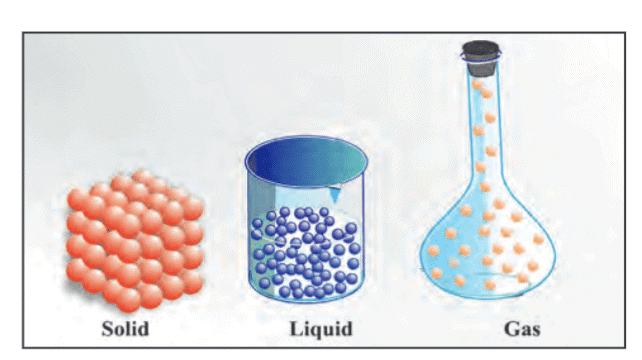
- States of Matter:
- Solid: Definite shape and volume, particles closely packed with limited movement.
- Liquid: Definite volume, no definite shape, particles move freely.
- Gas: No definite shape or volume, particles far apart and move fast.
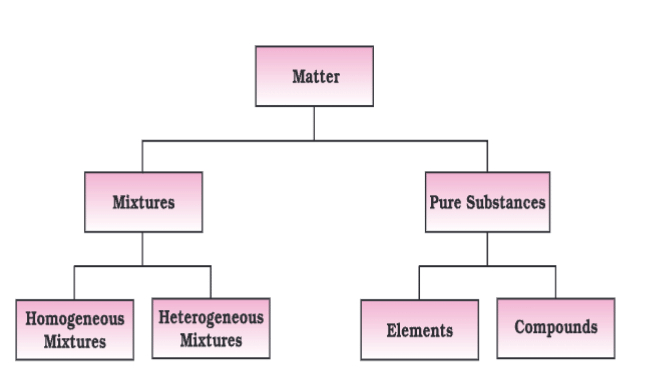
- Classification:
- Mixtures: Variable composition (homogeneous: uniform, e.g., sugar solution; heterogeneous: non-uniform, e.g., salt and sugar).
- Pure Substances: Fixed composition (elements: one type of atom, e.g., sodium; compounds: fixed ratio of atoms, e.g., water).
Properties of Matter and Their Measurement
- Types: Physical (e.g., color, density) measured without changing composition; Chemical (e.g., reactivity) require chemical change.
- SI Units:
- Key Measurements:
- Mass: Constant, measured in kg or g (e.g., analytical balance).
- Volume: Space occupied, in m³, cm³, or L (e.g., graduated cylinder).
- Density: Mass/Volume, in kg m⁻³ or g cm⁻³.
- Temperature: Scales (°C, °F, K), K = °C + 273.15.
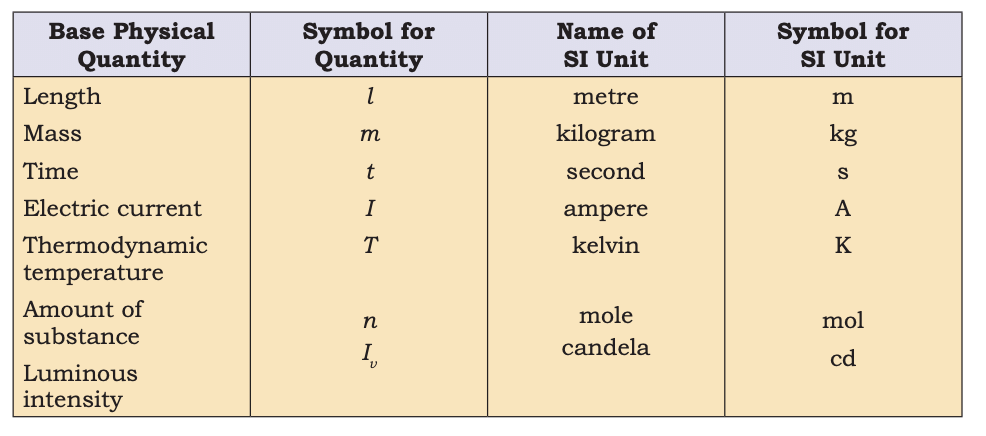 Base Physical Quantities and their Units
Base Physical Quantities and their Units
Uncertainty in Measurement
- Scientific Notation: Expresses large/small numbers (e.g., 232.508 = 2.32508 × 10²).
- Significant Figures: Certain digits + one uncertain (e.g., 11.2 mL: 11 certain, 2 uncertain).
- Rules: Non-zero digits significant, zeros between non-zeros significant, trailing zeros after decimal significant.
- Precision vs. Accuracy: Precision (closeness of measurements), Accuracy (closeness to true value).
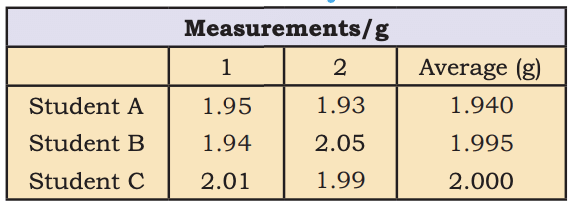 Data to Illustrate Precisionand Accuracy
Data to Illustrate Precisionand Accuracy - Dimensional Analysis: Converts units (e.g., 3 in = 7.62 cm using 1 in = 2.54 cm).
Laws of Chemical Combination
- Law of Conservation of Mass: Mass of reactants equals mass of products.

- Law of Definite Proportions: Fixed ratio of elements in a compound.

- Law of Multiple Proportions: Elements combine in simple whole-number ratios.
- Gay Lussac’s Law: Gaseous volumes in simple ratios.

- Avogadro Law: Equal volumes of gases contain equal numbers of molecules.

Dalton’s Atomic Theory
In 1808, Dalton published the New System of Chemical Philosophy, in which he proposed the following :
1. Matter consists of indivisible atoms.
2. All atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reactions involvethe reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
Mole Concept and Molar Masses
- Mole: 6.022 × 10²³ entities (Avogadro constant), mass in grams = molar mass (e.g., H₂O = 18.02 g mol⁻¹).
- Calculations: Relates mass, moles, and number of particles.
Percentage Composition
- Mass % = (Mass of element/Molar mass) × 100 (e.g., H₂O: H = 11.18%, O = 88.79%).
- Empirical Formula: Simplest ratio (e.g., CH₂Cl from 4.07% H, 24.27% C, 71.65% Cl).
- Molecular Formula: Exact number, uses molar mass (e.g., C₂H₄Cl₂, molar mass 98.96 g).
 One mole of various substances
One mole of various substances
Stoichiometry and Stoichiometric Calculations
- Balanced Equation: e.g., CH₄ + 2O₂ → CO₂ + 2H₂O; coefficients show molar ratios.
- Limiting Reagent: Reactant consumed first limits product (e.g., H₂ in N₂ + 3H₂ → 2NH₃).
- Solution Concentration:
- Mass %: (Mass solute/Mass solution) × 100.
- Mole Fraction: Moles component/Total moles.
- Molarity (M): Moles solute/L solution.
- Molality (m): Moles solute/kg solvent.
|
114 videos|263 docs|74 tests
|
FAQs on Revision Notes: Some Basic Concepts of Chemistry - Chemistry Class 11 - NEET
| 1. What are the basic concepts of chemistry that are essential for NEET preparation? |  |
| 2. How does the periodic table help in understanding chemical properties? |  |
| 3. What is stoichiometry and why is it important in chemistry? |  |
| 4. Can you explain the concept of moles and their significance in chemistry? |  |
| 5. What are the common types of chemical bonds and their characteristics? |  |

















