NEET Exam > NEET Notes > Chemistry Class 11 > Revision Notes: Classification of Elements & Periodicity in Properties
Revision Notes: Classification of Elements & Periodicity in Properties | Chemistry Class 11 - NEET PDF Download
Introduction
The periodic table organizes elements based on their properties, evolving from early classifications to a systematic arrangement reflecting atomic structure and periodicity in physical and chemical properties.
Genesis of Periodic Classification
- Johann Dobereiner (1829): Proposed Triads—groups of three elements with the middle element’s atomic weight averaging the other two and properties intermediate (e.g., Li, Na, K).
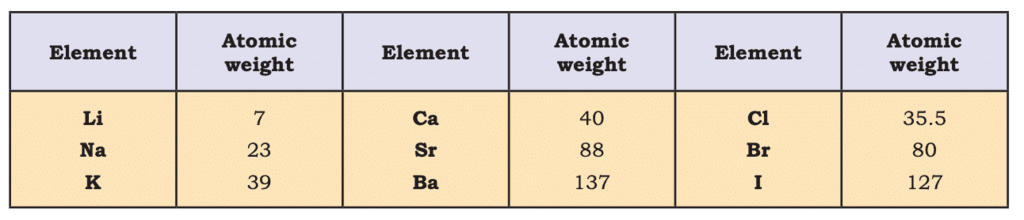 Dobereiner’s Triads
Dobereiner’s Triads - A.E.B. de Chancourtois (1862): Arranged elements by increasing atomic weights on a cylindrical table, showing periodic recurrence.
- John Newlands (1865): Law of Octaves—every eighth element repeats properties, valid up to calcium (e.g., Li to F, Na to Cl).
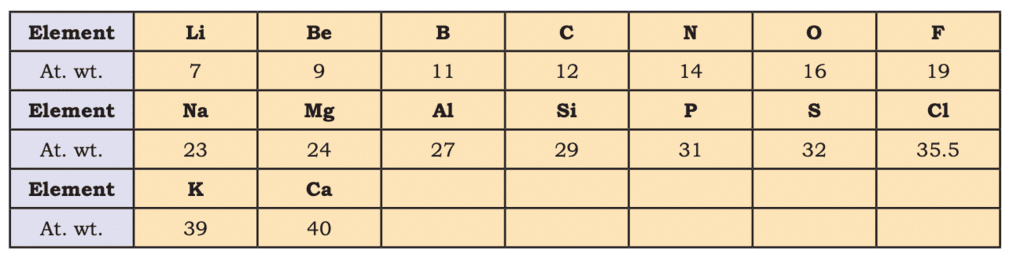 Newlands’ Octaves
Newlands’ Octaves - Lothar Meyer (1869): Plotted physical properties (e.g., atomic volume) vs. atomic weight, noting periodic patterns.
- Dmitri Mendeleev (1869): Formulated Periodic Law—“Properties of elements are periodic functions of their atomic weights.” Arranged elements in a table, prioritizing similar properties over strict atomic weight order, predicting undiscovered elements (e.g., Eka-aluminium, Eka-silicon).
Mendeleev’s Periodic Table
- Arranged elements by increasing atomic weights, grouping those with similar properties (e.g., Iodine with halogens despite lower atomic weight than Tellurium).
- Predicted properties of undiscovered elements (e.g., gallium, germanium), validated later:
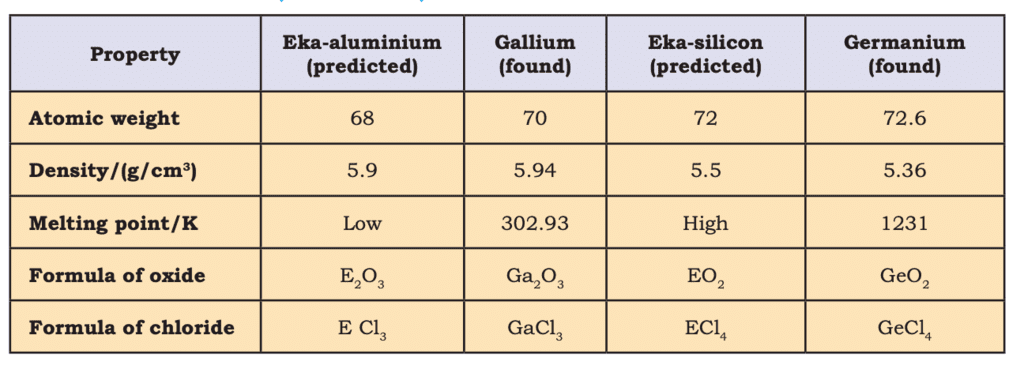 Mendeleev’s Predictions for the Elements Eka-aluminium (Gallium) andEka-silicon (Germanium)
Mendeleev’s Predictions for the Elements Eka-aluminium (Gallium) andEka-silicon (Germanium)
Modern Periodic Law and Table
- Henry Moseley (1913): Showed atomic number (Z) as the fundamental property via X-ray spectra, leading to Modern Periodic Law—“Properties are periodic functions of atomic numbers.”
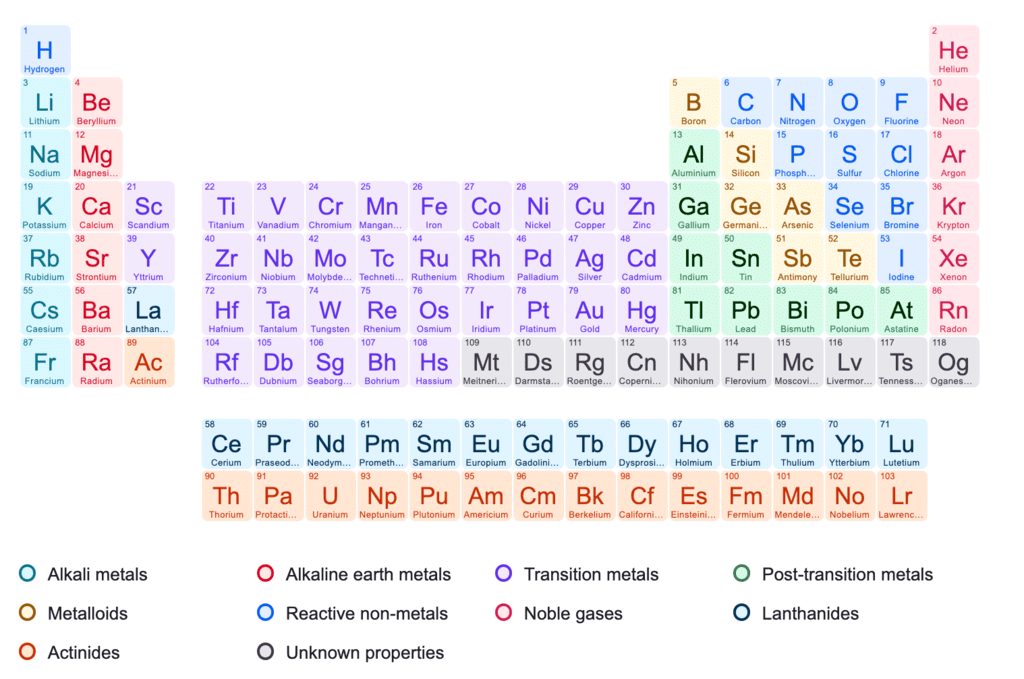
- Structure: 7 periods (rows), 18 groups (columns); periods reflect principal quantum number (n), groups reflect similar valence electron configurations.
- Periods: 1 (2 elements), 2-3 (8 each), 4-5 (18 each), 6-7 (32 each, 7th incomplete).
- Groups: Numbered 1-18 (IUPAC); lanthanoids and actinoids placed separately.
Nomenclature of Elements (Z > 100)
- IUPAC systematic naming uses numerical roots until discovery is confirmed (e.g., Z=120: unbinilium, Ubn).
- Notation:
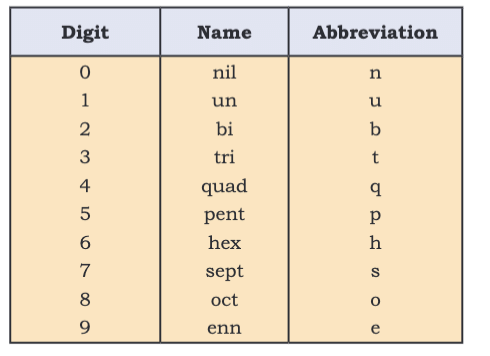
- Examples: Z=106 (Seaborgium, Sg), Z=114 (Flerovium, Fl).
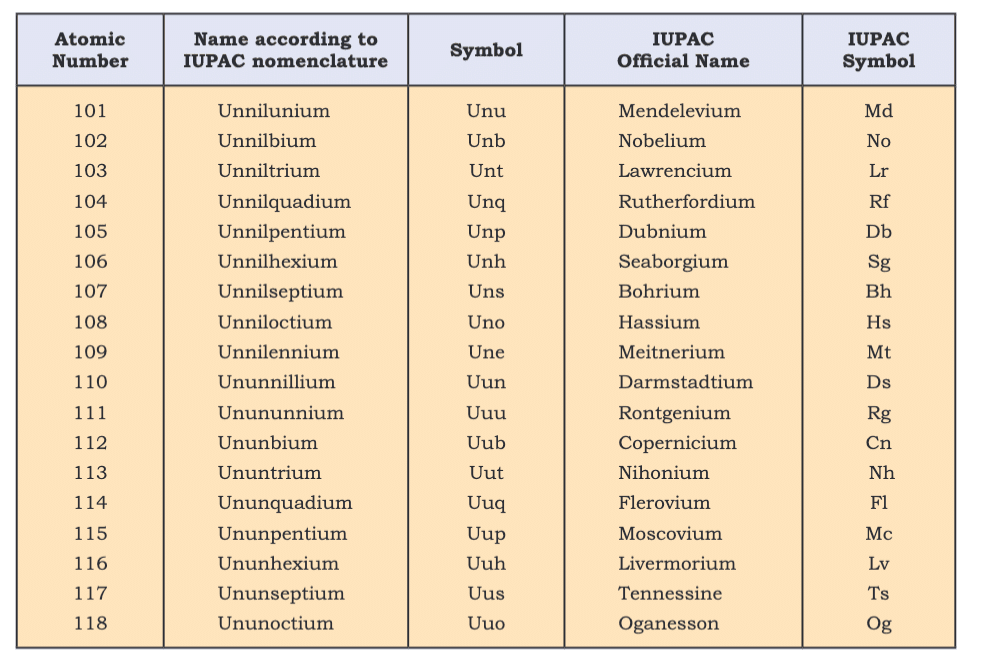
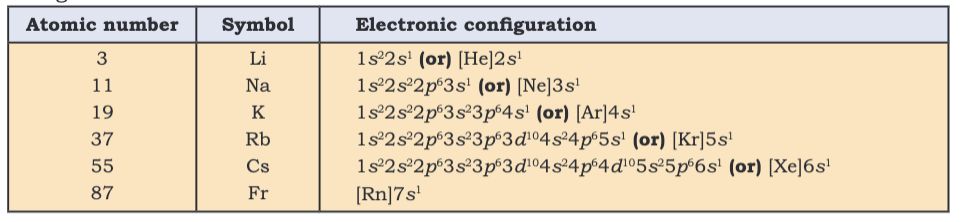
Electronic Configurations and Periodic Table
- Periods: Reflect filling of shells (n); e.g., Period 1 (1s, 2 elements), Period 4 (4s, 3d, 4p, 18 elements).
- Groups: Similar valence configurations (e.g., Group 1: ns¹).
- Blocks:
- s-block: Groups 1-2, filling s-orbitals.
- p-block: Groups 13-18, filling p-orbitals.
- d-block: Groups 3-12, filling d-orbitals.
- f-block: Lanthanoids/Actinoids, filling f-orbitals.
- Exceptions: H (1s¹, unique), He (1s², with noble gases).
Types of Elements
- Metals: >78% of elements, left side, high melting/boiling points, conductors, malleable, ductile.
- Non-metals: Top-right, low melting/boiling points, poor conductors, brittle.
- Metalloids: Borderline (e.g., Si, Ge), mixed properties.
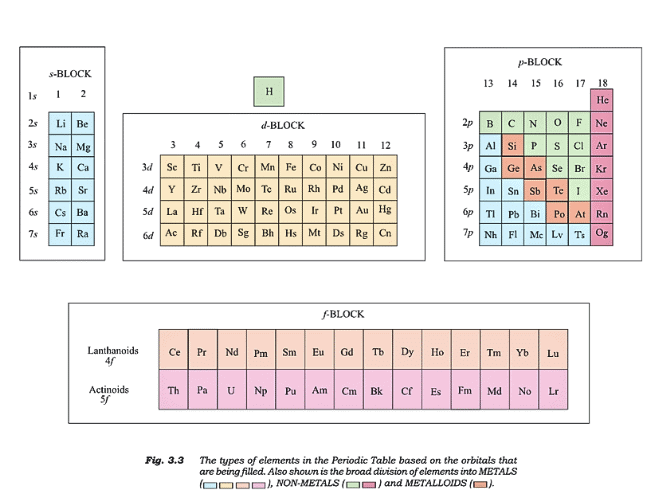
- Trends: Metallic character increases down groups, decreases across periods.
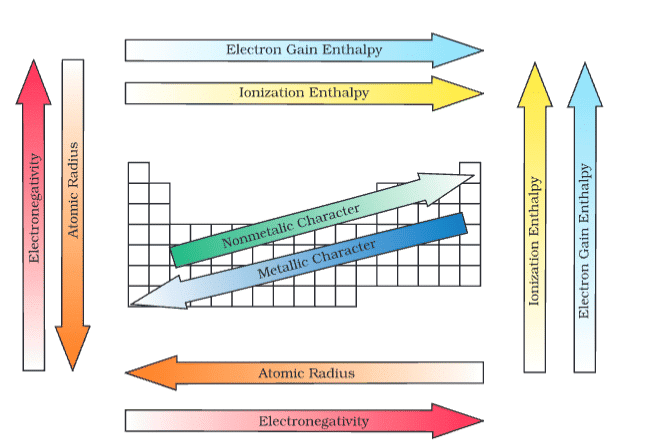
Periodic Trends in Physical Properties
Atomic Radius:
- Decreases across period (increased nuclear charge); e.g., Li (152 pm) to F (64 pm).
- Increases down group (more shells); e.g., Li (152 pm) to Cs (262 pm).
Ionic Radius:
- Cations smaller (e.g., Na⁺ 95 pm vs. Na 186 pm), anions larger (e.g., F⁻ 136 pm vs. F 64 pm);
- isoelectronic species vary by nuclear charge (e.g., Mg²⁺ < Na⁺ < F⁻).
Ionization Enthalpy (ΔᵢH):
- Increases across period (e.g., Na 496 to Si 786 kJ mol⁻¹), decreases down group (e.g., Li to Cs).
- Exceptions: B < Be (s vs. p), O < N (electron repulsion).
Electron Gain Enthalpy (Δₑ₉H):
- More negative across period (e.g., F -328 kJ mol⁻¹), less negative down group (e.g., Cl -349 to I -295 kJ mol⁻¹).
- Exceptions: O, F less negative than S, Cl (repulsion in n=2).
Electronegativity:
- Increases across period (e.g., Li 1.0 to F 4.0), decreases down group (e.g., F 4.0 to At 2.2).
Periodic Trends in Chemical Properties
- Valence/Oxidation State: Equals valence electrons or 8 minus valence electrons (e.g., Group 1: 1, Group 17: 1 or 7); e.g., OF₂ (O +2), Na₂O (Na +1, O -2).
- Anomalous Properties of Second Period: Li, Be, B to F differ due to small size, high electronegativity, limited orbitals (max covalency 4); e.g., [BF₄]⁻ vs. [AlF₆]³⁻.
- Chemical Reactivity: High at extremes (Group 1 loses e⁻, Group 17 gains e⁻); oxides basic (left, e.g., Na₂O) to acidic (right, e.g., Cl₂O₇), amphoteric/neutral in center (e.g., Al₂O₃, CO).
The document Revision Notes: Classification of Elements & Periodicity in Properties | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
114 videos|263 docs|74 tests
|
FAQs on Revision Notes: Classification of Elements & Periodicity in Properties - Chemistry Class 11 - NEET
| 1. What are the main classifications of elements in the periodic table? |  |
Ans. The elements in the periodic table are primarily classified into three categories: metals, nonmetals, and metalloids. Metals, which occupy the left side and the center of the table, are typically good conductors of heat and electricity. Nonmetals, located on the right side, are generally poor conductors and have varied properties. Metalloids, found between metals and nonmetals, exhibit properties of both categories.
| 2. What is periodicity in the context of the periodic table? |  |
Ans. Periodicity refers to the recurring trends in the properties of elements as you move across the periodic table. These trends include variations in atomic size, ionization energy, electron affinity, and electronegativity. Periodicity is a result of the arrangement of electrons in atoms and their interactions, leading to predictable patterns in chemical behavior.
| 3. How does atomic size change across a period and down a group in the periodic table? |  |
Ans. Atomic size decreases across a period from left to right due to the increasing nuclear charge, which pulls the electrons closer to the nucleus. Conversely, atomic size increases down a group because additional electron shells are added, which outweighs the effect of increased nuclear charge, resulting in larger atomic radii.
| 4. What are the trends in ionization energy across periods and down groups? |  |
Ans. Ionization energy generally increases across a period from left to right because of the increasing nuclear charge and greater attraction between the nucleus and the outer electrons. In contrast, ionization energy decreases down a group, as the outer electrons are farther from the nucleus and experience increased shielding from inner electrons, making them easier to remove.
| 5. How do electronegativity values change in the periodic table? |  |
Ans. Electronegativity tends to increase across a period from left to right, as elements become more effective at attracting electrons due to increased nuclear charge. It decreases down a group because the distance between the nucleus and the outermost electrons increases, and the shielding effect makes it harder for the nucleus to attract bonding electrons.
Related Searches





















