Mnemonics: Equilibrium | Chemistry Class 11 - NEET PDF Download
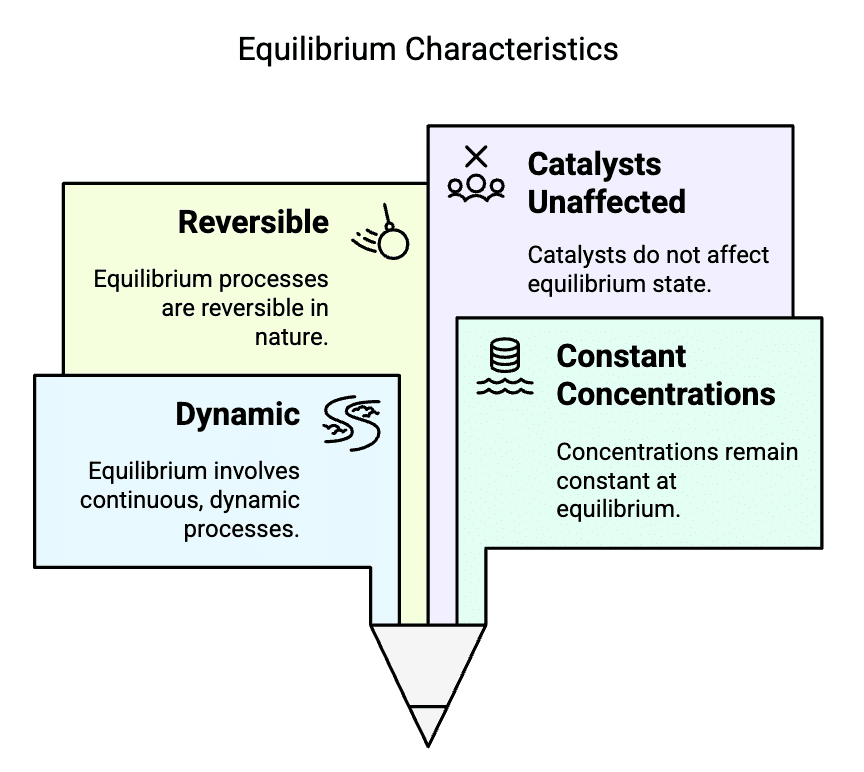
Characteristics of Equilibrium
Types: Dynamic, Constant Concentrations, Reversible, Catalysts Unaffected
Mnemonic: "Diya Chats, Ravi Cheers"
Breakdown:
- Diya - Dynamic (forward and backward)
- Chats - Constant Concentrations (At equilibrium, the concentrations of reactants and products remain constant over time.)
- Ravi - Reversible
- Cheers - Catalysts Unaffected (Don't affect position of equilibrium)
Factors Affecting Equilibrium (Le Chatelier’s Principle)
Types: Concentration, Pressure, Temperature
Mnemonic: "Charlie Pushes Tom"
Breakdown:
- Charlie - Concentration
- Pushes - Pressure
- Tom - Temperature
Explanation: Altering any of these factors will affect position of equilibrium.
Equilibrium Constant Expressions
Types: Kc (Concentration), Kp (Pressure)
Mnemonic: "Karan Calls Priya"
Breakdown:
- Karan Calls - Kc (In cases of reactants or products)
- Kanu Priya - Kp (In cases of gases)
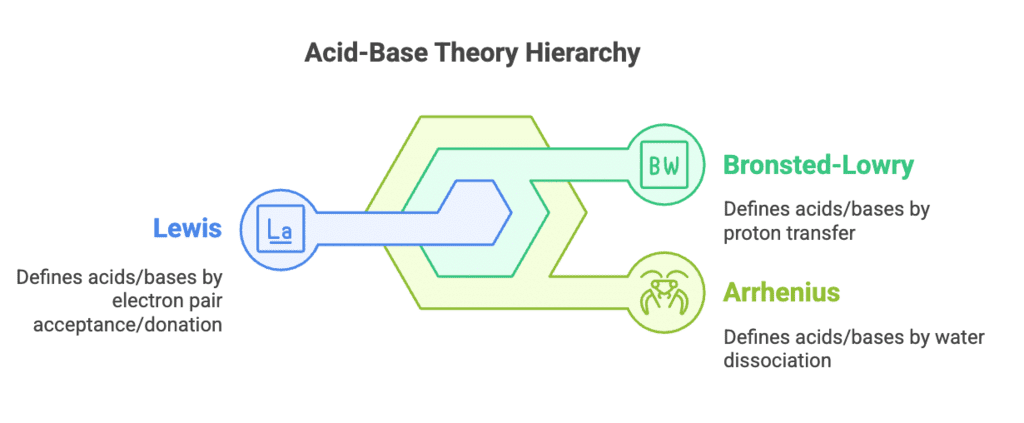
Acid-Base Equilibrium Concepts
Types: Arrhenius, Bronsted-Lowry, Lewis
Mnemonic: "Amit Bakes Lemonade-pie"
Breakdown:
- Amit - Arrhenius Theory
- Bakes - Bronsted-Lowry Theory
- Lemonade-pie - Lewis Theory
Hydrolysis of Salts
Mnemonic: "Salts Split, pH Shifts, Hydrolysis Drifts!"
Explanation:
Salts Split = Salts dissociate into ions in water.
pH Shifts = The pH of the solution can be acidic or basic depending on the salt.
Hydrolysis Drifts = Hydrolysis refers to the reaction between water and the ions of the salt, causing pH changes.
Ionic Equilibrium
Mnemonic: "Ions Dissociate, Buffers Regulate, Common Ions Slow!"
Explanation:
Ions Dissociate = Refers to the ionization of acids, bases, and salts.
Buffers Regulate = Buffers maintain pH stability in a solution.
Common Ions Slow = Common-ion effect decreases ionization in a solution.
Solubility Equilibrium Components
Types: Solubility Product (Ksp), Common Ion Effect, Precipitation
Mnemonic: "Sonia Picks Cakes"
Breakdown:
- Sonia - Solubility Product (Ksp-constant)
- Picks - Precipitation (Ksp dependent)
- Cakes - Common Ion Effect (Affects solubility)
Equilibrium Concentration Calculations
Mnemonic:
"K is Key, Concentrations Flow, Solve Step-by-Step!"
Explanation:
K is Key = The equilibrium constant is key to solving concentration problems.
Concentrations Flow = Concentration of reactants and products are used to find the equilibrium concentrations.
Solve Step-by-Step = Apply stoichiometric relationships and K to calculate equilibrium concentrations.
|
114 videos|263 docs|74 tests
|
FAQs on Mnemonics: Equilibrium - Chemistry Class 11 - NEET
| 1. What is the importance of equilibrium in chemistry for the NEET exam? |  |
| 2. How can I effectively memorize the different types of chemical equilibria for the NEET exam? |  |
| 3. What are Le Chatelier's principles and how do they apply to NEET questions? |  |
| 4. What role does temperature play in shifting the position of equilibrium, and how can I prepare for related NEET questions? |  |
| 5. Are there any common mistakes students make regarding equilibrium concepts in the NEET exam? |  |
















