Grade 7 Exam > Grade 7 Notes > Science for Grade 7 > Revision Notes: Elements Compounds Symbols and Formulae
Revision Notes: Elements Compounds Symbols and Formulae | Science for Grade 7 PDF Download
Elements and Compounds
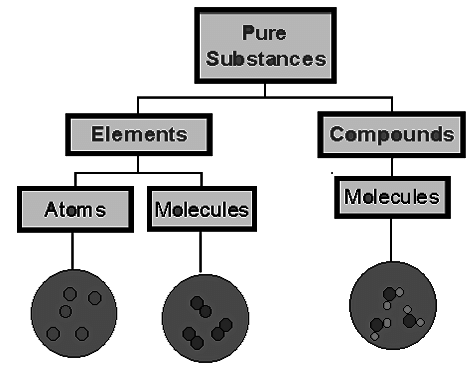
Elements can be further divided as metals, non-metals, metalloids and noble gases
Atoms: The smallest particle of an element which normally does not exist independently
Molecule: The smallest particle of an element or compound that can exist independently Molecules of elements are made of identical atoms held by bonds Molecules of compounds are made of different kinds of atoms bound in a definite ratio Molecular formula represents the composition of a molecule of an element or a compound Atomicity is the number of atoms seen in a molecule e.g.
Monoatomic: Helium He, Neon Ne, Argon Ar.
These are noble gases
Diatomic: Oxygen O2, Hydrogen H2, Nitrogen N2
Triatomic: Ozone O3
Polyatomic: Sulphur S8, Phosphorus P4
Symbols of Some Pure Elements
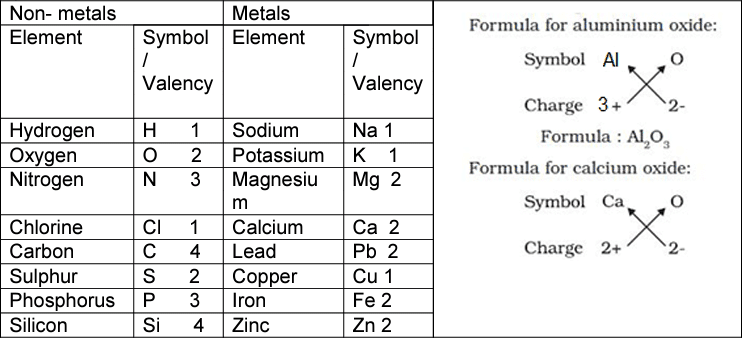
The document Revision Notes: Elements Compounds Symbols and Formulae | Science for Grade 7 is a part of the Grade 7 Course Science for Grade 7.
All you need of Grade 7 at this link: Grade 7
|
64 docs|10 tests
|
FAQs on Revision Notes: Elements Compounds Symbols and Formulae - Science for Grade 7
| 1. What are elements and why are they important in chemistry? |  |
Ans.Elements are pure substances that cannot be broken down into simpler substances by chemical means. They consist of only one type of atom, and each element has unique properties. Elements are important in chemistry because they are the building blocks of all matter. Understanding elements allows us to comprehend how substances interact, combine, and change during chemical reactions.
| 2. How are compounds different from elements? |  |
Ans.Compounds are substances formed when two or more elements chemically combine in fixed proportions. Unlike elements, which contain only one type of atom, compounds consist of molecules made up of different atoms. The properties of a compound can be very different from the properties of the individual elements that compose it. For example, sodium (a metal) and chlorine (a gas) combine to form sodium chloride (table salt), which is a solid.
| 3. What are chemical symbols and how are they used? |  |
Ans.Chemical symbols are one or two-letter abbreviations used to represent elements on the periodic table. For example, the symbol for hydrogen is "H," and the symbol for oxygen is "O." These symbols allow chemists to write formulas and equations concisely, making it easier to communicate complex chemical information. Each symbol is unique to an element and is derived from its name, often in Latin or Greek.
| 4. How do you write a chemical formula for a compound? |  |
Ans.A chemical formula represents the elements in a compound and the number of atoms of each element present. To write a chemical formula, you start by identifying the elements in the compound and their respective quantities. For instance, in water (H₂O), there are two hydrogen atoms and one oxygen atom. The formula reflects this by using subscripts to indicate the number of atoms (H₂ for two hydrogen atoms and O for one oxygen atom).
| 5. What are some common examples of compounds, and what are their uses? |  |
Ans.Some common examples of compounds include water (H₂O), which is essential for life; carbon dioxide (CO₂), which is produced during respiration and used by plants for photosynthesis; and sodium chloride (NaCl), commonly known as table salt, used for seasoning food. Each of these compounds has unique properties and plays crucial roles in both biological and chemical processes.
Related Searches





















