Class 4 Exam > Class 4 Notes > Year 4 Science IGCSE (Cambridge) > Chapter Notes: Materials
Materials Chapter Notes | Year 4 Science IGCSE (Cambridge) - Class 4 PDF Download
| Table of contents |

|
| Materials, Substances, and Particles |

|
| How Do Solids and Liquids Behave? |

|
| Melting and Solidifying |

|
| Chemical Reactions |

|
Materials, Substances, and Particles
- Materials: Objects are made from various types of materials, such as wood, metal, plastic, rubber, and glass. All materials consist of matter, which includes everything around us. A material is a specific type of matter, often a mix of different substances. Example: A school desk may be made from two types of materials: wood and metal.
- Substances: A substance is a pure form of matter, not a mixture, and can be solid, liquid, or gas. Examples: Salt is a solid substance. Pure water is a liquid substance. Oxygen is a gaseous substance.
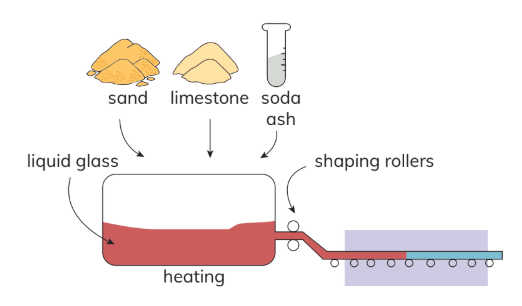
- Glass Production: Glass is a material created by mixing substances like sand, soda ash, and limestone. These substances are heated together to create a clear liquid glass. This liquid can be shaped into various items, such as window panes, bottles, and light bulbs. When cooled, the liquid glass hardens into a solid material.
- Particles: All matter is made up of particles, which are tiny parts of a substance. Some particles, like dust, can be seen, but most are too small to notice.
The Particle Model of Matter
- Particles have spaces between them: The amount of space between particles varies. In solids, the spaces are smaller, while in gases, the spaces are larger.
- Particles are always moving: Although we cannot see this movement, particles are constantly in motion. The speed of their movement varies depending on the state of matter.
- The amount of movement determines the state: The movement of particles is what differentiates solids, liquids, and gases. In solids, particles vibrate in fixed positions. In liquids, they move more freely, and in gases, they move rapidly and are far apart.
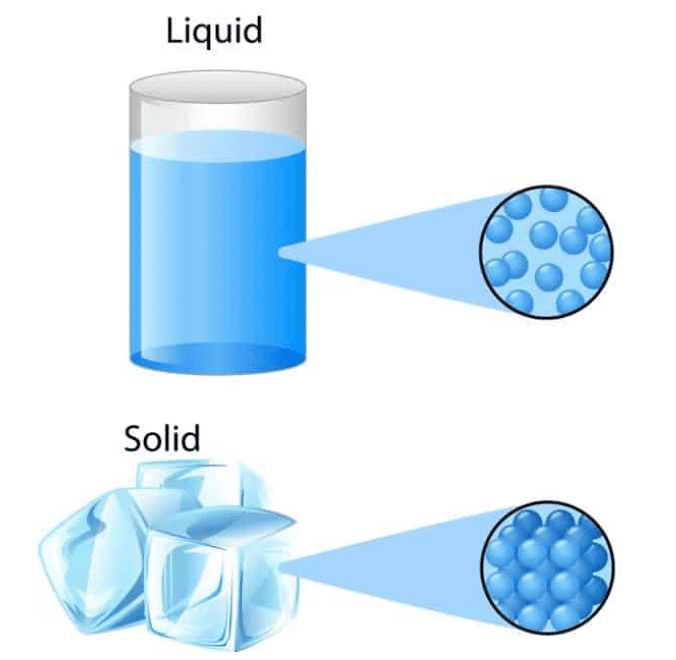
Particle Model for Solids
- In solids, particles are tightly packed in a regular pattern with little space between them.
- The small spaces limit particle movement, keeping them in fixed positions, which allows solids to hold their shape.
Particle Model for Liquids
- In liquids, particles are close together but not in a regular arrangement.
- Larger spaces between particles let them slide past each other and change places.
- This ability allows liquids to flow and take the shape of their container.
How Do Solids and Liquids Behave?
A property describes the characteristics or behaviour of a substance or material, which can be measured, seen, or felt. Solids and liquids have different properties due to their particle arrangements.
Properties of Solids
- Solids keep their shape unless an external force, like pressure or impact, is applied.
- The shape of a solid does not change on its own.
- Some solids can be reshaped with enough force, like squeezing or pushing.
- Particles in solids are tightly packed, preventing significant movement and maintaining a fixed shape.
Properties of Liquids
- Liquids do not hold a fixed shape; they flow and take the shape of their container.
- Particles in liquids are further apart than in solids, allowing more movement.
- This movement lets liquid particles slide past each other, enabling flow.
Can solids behave like liquids?
- Most solids are rigid and cannot flow because their particles are tightly packed with little room for movement. However, some solids, known as powders (e.g., sand, flour, salt, sugar), can behave like liquids.
- Powders can be poured and take the shape of their container, similar to liquids.
- Powders consist of tiny grains with air spaces between them, allowing grains to move and flow.
- Each grain is made of millions of microscopic particles that are too small to see.
Melting and Solidifying
Change of state
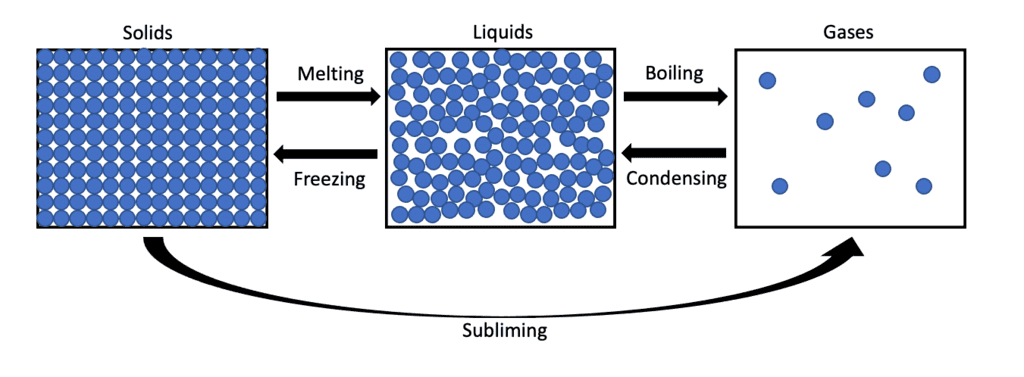
- A change of state refers to the process where materials or substances transition between different forms, namely solid, liquid, and gas, when subjected to heating or cooling.
- These forms represent different states of matter.
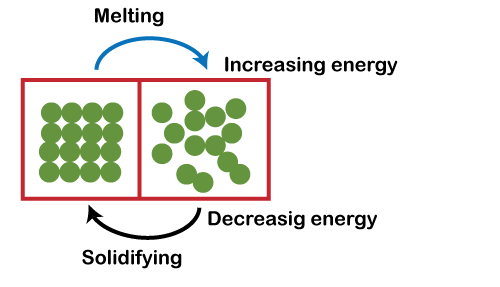
Melting
- Melting is the process of changing a substance from solid to liquid due to the application of heat.
- For instance, ice-cream transforms from solid to liquid when warmed by the Sun.
- Representation: solid → heating → liquid (melting).
Solidifying
- Solidifying, also known as freezing, is the process of changing a substance from liquid to solid as a result of cooling.
- Liquids lose heat, which leads to their solidification.
- Representation: liquid → cooling → solid (solidifying).
- Some liquids, such as water, require significant cooling to solidify, hence the term "freezing."
Physical Process
- A change of state, encompassing processes like melting and solidifying, is classified as a physical process. This means it only alters the form of the substance, not its chemical composition.
- The substance remains the same despite undergoing a change in state. For example, when ice melts into water or when water freezes into ice, the substance involved is always H₂O.
Change of State and the Particle Model
The particle model elucidates changes of state by detailing variations in particle movement and arrangement:
- In solids: Particles are closely packed and vibrate within fixed positions, exhibiting minimal movement.
- Melting Process: Upon heating, particles acquire energy, converting heat energy into kinetic energy. This increase in energy results in faster and more extensive movement of particles. When particles gain sufficient energy, they overcome their fixed positions, leading to the melting process.
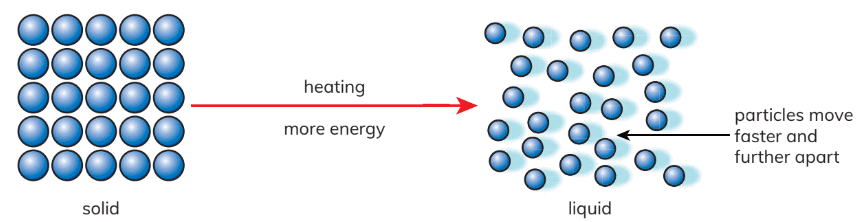
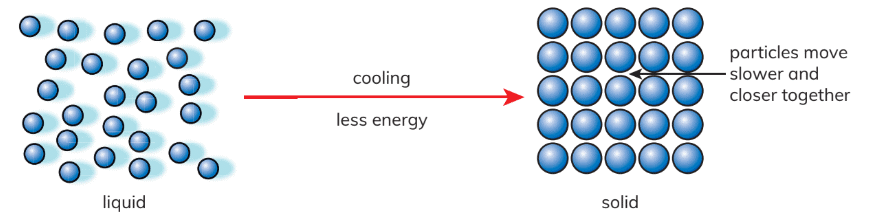
- In liquids: Particles are not as tightly packed and possess the ability to slide past one another.
- Solidifying Process: During cooling, particles lose energy, resulting in a decrease in their movement and bringing them closer together, thereby facilitating the formation of a solid.
Melting in Different Solids
- Various solids exhibit different melting rates based on their intrinsic properties.
- For example, metals like gold and silver can be melted, but they require extremely high temperatures for this process.
- When these melted metals cool down, they revert to their solid state.
Chemical Reactions

Making new substances
- A chemical reaction takes place when substances are combined and react to form new substances or materials. Unlike physical processes such as a change of state, chemical reactions lead to the creation of new substances with distinct properties.
- One of the key features of chemical reactions is their irreversibility. The original substances involved in the reaction cannot be easily restored to their former state. Examples of chemical reactions are commonly observed in everyday life.
Examples of Chemical Reactions
- Concrete Formation: Builders mix cement powder with sand, water, and limestone to create concrete, a new material that cannot revert to its original components.
- Burning Wood: When wood is heated, it reacts with oxygen in the air, producing ash (a solid) and gases. Ash and gases are new substances different from the original wood.
- Rusting: Rusting is a chemical reaction where metal (e.g., in tin cans or cars) reacts with oxygen and water to form rust, a reddish-brown substance. Rust is a new substance that weakens the metal structure.
The document Materials Chapter Notes | Year 4 Science IGCSE (Cambridge) - Class 4 is a part of the Class 4 Course Year 4 Science IGCSE (Cambridge).
All you need of Class 4 at this link: Class 4
|
14 docs|7 tests
|
FAQs on Materials Chapter Notes - Year 4 Science IGCSE (Cambridge) - Class 4
| 1. What is the particle model of matter and how does it explain the properties of solids and liquids? |  |
Ans. The particle model of matter is a scientific theory that describes how all matter consists of tiny particles that are in constant motion. In solids, these particles are closely packed together in a fixed arrangement, which gives solids a definite shape and volume. In liquids, the particles are still close together but can move past one another, allowing liquids to take the shape of their container while maintaining a fixed volume. This model helps explain the different properties of solids and liquids, such as rigidity in solids and fluidity in liquids.
| 2. What are the key properties that differentiate solids from liquids? |  |
Ans. The key properties that differentiate solids from liquids include shape, volume, and particle arrangement. Solids have a definite shape and volume because their particles are tightly packed in a fixed arrangement. Liquids, on the other hand, have a definite volume but take the shape of their container, as their particles are less tightly packed and can move around each other. Additionally, solids are generally incompressible, while liquids can be slightly compressed.
| 3. Can solids behave like liquids under certain conditions? |  |
Ans. Yes, some solids can behave like liquids under certain conditions. This phenomenon is known as "non-Newtonian behavior." For example, materials like cornstarch mixed with water (oobleck) can act like a solid when stressed or compressed but flow like a liquid when at rest. Additionally, certain solids can melt and flow when heated, transitioning to a liquid state.
| 4. What is meant by a change of state in matter, and what are some examples? |  |
Ans. A change of state in matter refers to the process by which a substance transitions from one state of matter (solid, liquid, or gas) to another due to changes in temperature or pressure. Examples include melting (solid to liquid), freezing (liquid to solid), evaporation (liquid to gas), and condensation (gas to liquid). This process involves changes in the arrangement and energy of the particles.
| 5. How does the particle model explain changes of state in materials? |  |
Ans. The particle model explains changes of state by describing how the energy of the particles changes during the transition. For instance, when a solid is heated, the particles gain energy, vibrate more, and may overcome the forces holding them in place, resulting in melting. Conversely, when a gas cools, the particles lose energy and slow down, allowing them to come closer together and condense into a liquid. This model provides a clear understanding of how temperature and energy affect the behavior of particles during changes of state.
Related Searches















