Class 9 Exam > Class 9 Notes > Year 9 Science IGCSE (Cambridge) > Chapter Notes: Forces and Energy
Forces and Energy Chapter Notes | Year 9 Science IGCSE (Cambridge) - Class 9 PDF Download
| Table of contents |

|
| Density |

|
| Heat and Temperature |

|
| Conservation of Energy |

|
| Moving from Hot to Cold |

|
| Ways of Transferring Thermal Energy |

|
| Cooling by Evaporation |

|
Density
Mass and Volume
 Material Properties
Material Properties
- Density is a measure of how mass is distributed within a given volume of a material.
- It indicates the amount of mass contained in a specific volume.
- When an object has high density, it means there is a lot of mass packed into a small volume.
- Conversely, low density means there is less mass in a larger volume.
Calculating volume
- People often assume that rocks or metals are heavier than materials like feathers or polystyrene, but this assumption is based on how mass and volume interact.
- For example, a large piece of polystyrene can actually weigh more than a small piece of metal.
- Polystyrene food containers feel light because they have a large volume with low mass; they are mostly hollow and filled with air.
- On the other hand, iron nails feel heavy because they have high mass packed into a small volume; they are solid with no air inside.
- The term 'solid' refers to materials that do not have internal air spaces, in contrast to hollow materials.
- While a specific polystyrene container might weigh more than a particular iron nail, in general, the same volume of iron weighs more than the same volume of polystyrene.
- Density is what explains this difference in mass per unit volume.
Calculating density
 Water Displacement
Water Displacement
- Regular Blocks: To find the volume of a regular block of material, measure the lengths of its sides and multiply them together.
- Volume Formula: Regular shapes have specific formulas for calculating volume. For example, a block of wood with sides measuring 10 mm, 6 mm, and 4 mm has a volume calculated as: Volume = 10 × 6 × 4 = 240 mm³. Since the side lengths are in millimeters, the unit of volume is mm³.
- Irregular Shapes - Displacement Method: For irregular shapes, the displacement method is used. This involves fully submerging the object in water and measuring the increase in water volume, which equals the object's volume.
- Example of Displacement: If a rock is placed in a cylinder containing 40 cm³ of water and the level rises to 56 cm³, the volume of the rock is calculated as: Volume = 56 cm³ - 40 cm³ = 16 cm³. In this case, the unit of volume is cm³ because the measuring cylinder uses cm³.
Comparing densities
 Material Density
Material Density
- Density is a measure of how much mass is contained in a given volume. It is calculated using the formula: density = mass / volume .
- For instance, consider a 1 cm³block of iron and a block of polystyrene:
- Iron : Mass = 7.9 g, Density = 7.9 g/cm³
- Polystyrene : Mass = 0.05 g, Density = 0.05 g/cm³
- The units of density vary based on the measurement of mass and volume. Common units include g/cm³ (grams per cubic centimeter) and kg/m³ (kilograms per cubic meter) .
Examples of Density in Different Materials
- Helium: 0.00018 g/cm³ - Very low density due to widely spaced particles.
- Air: 0.0012 g/cm³ - Low density, as air particles are also spread out.
- Wood: 0.35–0.95 g/cm³ - Density varies depending on the type of wood and how closely its particles are packed.
- Water: 1.0 g/cm³ - Standard density for liquids, with particles more closely packed than in gases.
- Concrete: 2.4 g/cm³ - Higher density due to tightly packed particles in this solid.
- Aluminium: 2.7 g/cm³ - Density of this metal, which has closely packed atoms.
- Osmium: 22 g/cm³ - One of the densest elements, with very tightly packed atoms.
- Gases like helium and air have lower densities compared to liquids and solids because their particles are more spread out. Density increases when particles are packed more closely together, which is why solids generally have the highest densities.
- For elements, density usually increases with atomic number. For example, osmium (atomic number 76) is denser than iron (atomic number 26) .
- Neutronium is a hypothetical substance found in collapsed stars, theorized to have an extremely high density of about 100,000,000,000,000 g/cm³. This is due to the extreme compression of atoms in such environments. A teaspoon of neutronium, based on these models, could weigh around 500 million tonnes.
Floating and Sinking
 Layered Serenity
Layered Serenity
- The ability of a material to float or sink in water is determined by its density in comparison to water's density, which is 1.0 g/cm³.
- Materials that are denser than water, such as aluminium and osmium, will sink. In contrast, materials that are less dense than water, like wood and helium, will float.
- Objects like pool floats, which are often made from materials like polystyrene or are hollow and filled with air, have a low density, allowing them to float easily.
- Despite steel having a high density of 8–9 g/cm³, steel ships can float due to the principle of buoyancy. This principle is influenced by the ship's shape and design.
- The presence of large air-filled spaces within these ships reduces their average density to below 1.0 g/cm³, enabling them to float.
- Average density is calculated by dividing the total mass of the ship, including the air, by its total volume.
- When cargo, passengers, or fuel are added to a ship, its mass and density increase, which can affect its ability to float.
Liquids of Different Density
- When mixing liquids of different densities, the less dense liquids will float on top of the denser ones, creating distinct layers if they do not mix.
- For instance, a combination of vegetable oil, water with red ink, liquid soap, corn syrup, and honey will form separate layers based on their densities.
- Crude oil is less dense than water, which is why it floats on the surface of water. This characteristic poses significant environmental risks, particularly during oil spills, as the oil can wash up on beaches.
Gases and Liquids
 Density Dynamics
Density Dynamics
- Gases are less dense than liquids because their particles are spaced far apart.
- Raindrops fall since water is denser than air, making it heavier.
- In fizzy drinks, carbon dioxide gas rises through the liquid because both the gas and the balloon are less dense than air, creating bubbles.
Gases of Different Density
- Gases have lower densities than solids or liquids, but densities can vary among different gases.
- Helium, a low-density gas, is used in balloons which float because both the balloon and helium are less dense than air.
- Hydrogen is lighter than helium but is not used in balloons mainly due to safety reasons, not just density.
- Solids and liquids are hard to compress as their particles are close together; in contrast, gases can be easily compressed because their particles are far apart.
- When a gas is compressed, its density increases by packing the same number of particles into a smaller space, while expanding decreases density.
- Heat causes gases to expand, which lowers their density; for instance, hot air balloons float because the hot air inside is less dense than the colder air outside.
Heat and Temperature
 Heat Dynamics
Heat Dynamics
Heat
- Thermal energy is the energy possessed by the particles within a material.
- Thermal energy can be transferred between objects, stored in an object, and eventually spreads to the surroundings. It is measured in joules.
- When the thermal energy of an object increases, its particles move faster, which raises their energy. Heat is the total thermal energy of all the vibrating particles in an object.
- For instance, if you compare two glasses with the same volume of water at different temperatures, the glass with the higher temperature has particles that are moving faster. Thus, it has more total thermal energy (heat).
- Similarly, two glasses with the same temperature but different volumes show that the larger volume has more particles, leading to greater total thermal energy (heat), even with identical temperatures.
Temperature
- Temperature indicates the direction of thermal energy transfer, which always occurs from a higher temperature to a lower temperature.
- For example, when ice cream at -20 °C is placed in a room at 24 °C, thermal energy flows from the warmer air to the colder ice cream because of the 44 °C temperature difference.
- The greater the temperature difference between two objects, the faster the transfer of thermal energy.
- Temperature also reflects the average energy of particles in an object. In contrast, heat measures the total energy of all particles. This distinction makes temperature useful for comparing the energy of particles in different-sized objects or materials.
- For instance, hot soup and cold water contain different types and amounts of particles. However, if the soup is hotter, its particles have a higher average energy than those in the water.
Heat and temperature in a sparkler
- A sparkler burning at 1000 °C does not cause severe burns because a single spark has a very small mass and therefore contains fewer particles, resulting in less total thermal energy (heat).
- The significant temperature difference between the spark and the surrounding air causes rapid thermal energy transfer to the air, cooling the spark before it can reach the skin.
- As temperature decreases, the movement of particles slows down. Scientists, including Lord Kelvin, have theorised that at absolute zero (-273 °C), particles would cease all movement. However, reaching absolute zero is impossible; scientists have only managed to achieve temperatures just above this limit in laboratory settings.
- Heat refers to the total thermal energy of all particles in an object, while temperature measures the average energy of the particles within that object.
Conservation of Energy
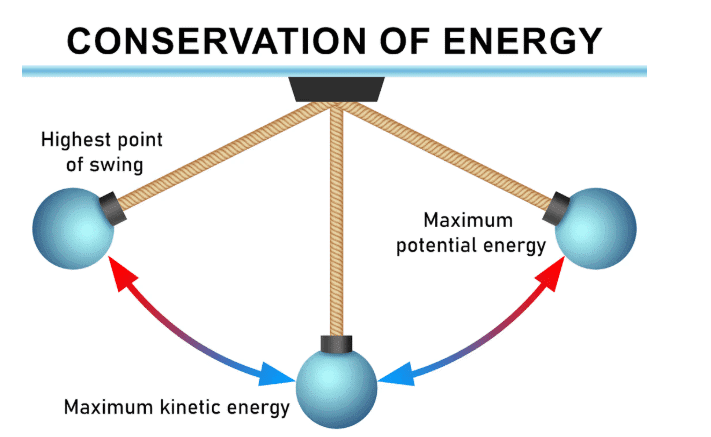
Energy is conserved
- Conserving energy in physics means keeping the total amount of energy the same, not necessarily using less energy.
- Energy is conserved when it is stored, changed, transferred, or dissipated. The total energy doesn’t increase or decrease.
- For instance, an electric lamp takes in 100 J of electrical energy and converts it into 10 J of light energy and 90 J of thermal energy, totaling 100 J. This demonstrates energy conservation.
- The light energy is useful, while the thermal energy is considered wasted.
- Similarly, a car engine converts 100% of the chemical energy in fuel into 25% kinetic energy, 35% thermal and sound energy, and 40% chemical energy in exhaust gas, also totaling 100%. This is another example of energy conservation.
- In physics, a system refers to an object or process being studied for energy changes, like a closed or open system such as the lamp or car engine.
- The total energy output in a system cannot exceed the total energy input because energy cannot be created or brought into existence.
- Wasted energy, like thermal energy, is dissipated, meaning it spreads to the surroundings and becomes less useful, but it is not destroyed.
- To destroy means to end something’s existence, which does not apply to energy.
- The law of conservation of energy states that energy cannot be created or destroyed, only changed or transferred.
Moving from Hot to Cold
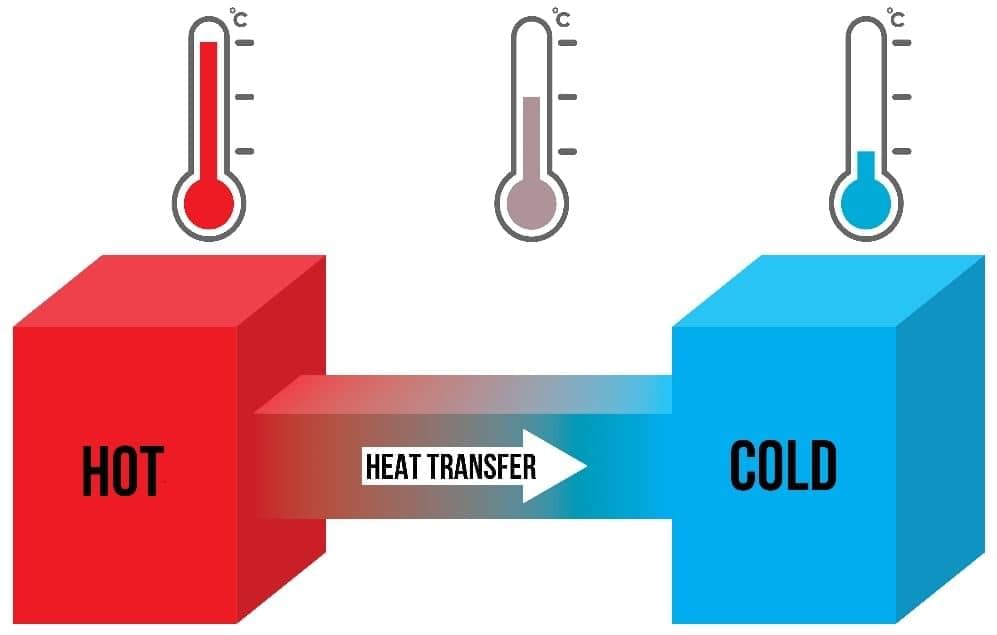
Feeling Heat
- Thermal energy always moves from hotter (higher temperature) to colder (lower temperature) places.
- For example, when you hold a hot drink, thermal energy flows from the hot drink, through the container, to your cooler hands, but only if the drink is hotter.
Feeling Cold
- Some applications of this principle include:
- In refrigerators, thermal energy is removed from food, cooling it down.
- Car engines use water to absorb thermal energy and prevent overheating.
- Animals cool off in water as thermal energy transfers from their bodies to the colder water.
- When thermal energy is taken away from a hot object, it is said to have dissipated.
- Holding ice feels cold because thermal energy is transferred from your hand to the ice, not because “cold” is moving; cold simply means less thermal energy.
- Prolonged contact with ice can damage skin because it removes too much thermal energy necessary for skin function.
- Opening a window in cold weather cools the room because thermal energy from the warmer indoor air moves to the colder outdoor air; cold air may enter, but the transfer is from warmer to colder air.
Dissipation
- Dissipation is about how energy spreads out and becomes less useful, like when thermal energy moves from a hot place to a cooler one.
- When thermal energy shifts from a hot spot to a cold one, we say it has dissipated from the hotter spot.
- The speed of thermal energy transfer goes up when there is a bigger temperature difference between the hot and cold areas.
- During dissipation, energy is not lost; thermal energy doesn’t disappear or get destroyed, it just spreads out to a colder area.
- Some researchers think that, billions of years from now, all thermal energy in the universe will be spread out, leading to a state of maximum entropy where energy changes are very small.
Ways of Transferring Thermal Energy
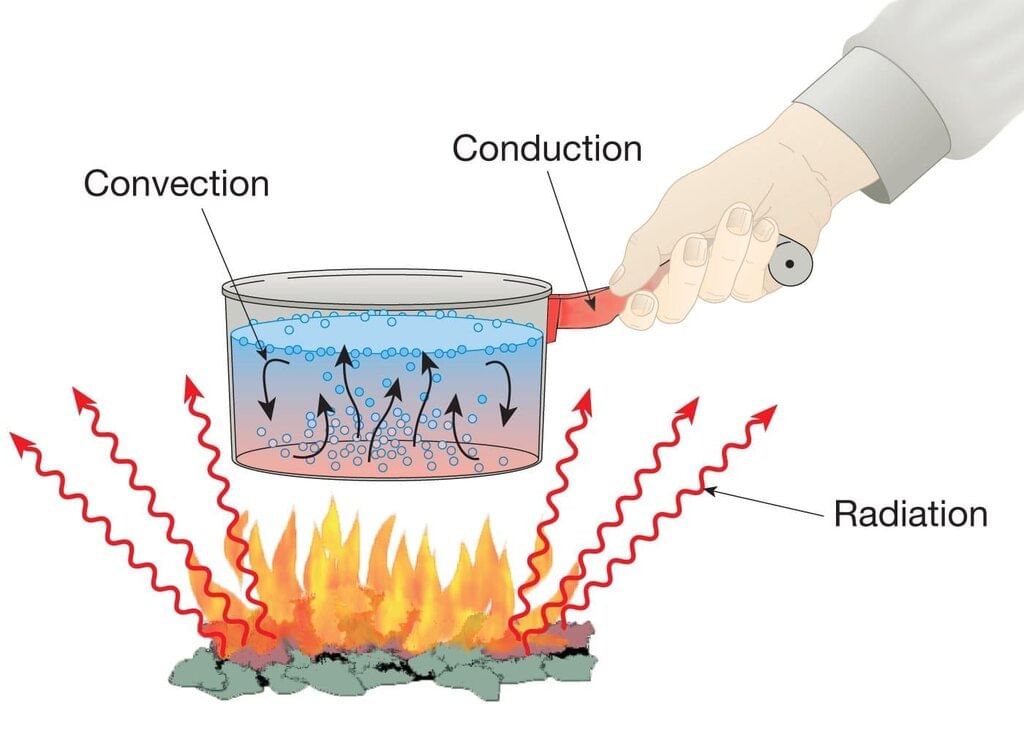
Thermal energy can move through conduction, convection, radiation, and processes like evaporation, each involving different ways particles behave.
Heat and Particle Movement
- When a substance is heated, the particles inside it start to move faster.
- In solids, the particles are like tiny balls stuck in place, but they can wiggle around a little. When you heat a solid, these particles wiggle faster and harder, and because they need more room to move around, the solid starts to expand.
- This faster wiggling means the particles have more energy, speed, and strength when they move.
- In liquids, the particles are not stuck in one spot; they can move around freely, but they still vibrate. Heating a liquid makes these particles vibrate and move faster, which also makes the liquid expand because the particles take up more space.
- In gases, the particles are far apart from each other and usually move in straight lines until they bump into something, like another particle or the walls of the container they are in. When you heat a gas, its particles move faster, and they hit each other and the walls more often and with more force, causing the gas to expand.
Conduction
- Conduction is the process of transferring thermal energy through vibrating particles. When one particle vibrates, it pushes against its neighboring particle, causing it to vibrate as well, and this continues throughout the material.
- Conduction is most effective in solids because the particles in solids are tightly packed together and can only vibrate in fixed positions. This makes it easier for the energy to be passed along from one particle to another.
- Metals are particularly good conductors of heat. This is due to their atomic structure and the presence of free-moving electrons. These free electrons can move through the metal and help transfer the vibrational energy more efficiently.
- On the other hand, materials like wood, plastics, and fabrics such as wool and cotton are poor conductors of heat. This is because their particles are not as closely packed, and they do not have free electrons to help with the transfer of energy. This property makes them suitable for use as thermal insulators.
- Insulators are materials that resist the transfer of thermal energy. Unlike conductors, they do not allow heat to pass through them easily.
- For example, a metal pan conducts thermal energy very well, which is why it heats up quickly and cooks food. However, the handle of the pan, which is often made of plastic, does not conduct heat as well. This is why the handle remains cool to the touch, making it safe to handle the pan. Similarly, a wooden spoon also acts as an insulator because wood does not conduct heat very well.
- In cold weather, clothes are designed using insulating materials that help reduce the loss of thermal energy from the body. These materials trap the heat and keep the body warm.
- Conduction is not as effective in liquids because, in liquids, the heated particles do not just vibrate; they also move around. This movement makes it harder for the energy to be transferred from one particle to another. In gases, conduction is even less effective because the particles are much further apart and do not collide with each other as often. This makes it difficult for the energy to be passed along. Conduction also cannot occur in a vacuum because there are no particles present to carry out the transfer of energy.
Convection
- Convection is a process that occurs in liquids and gases when heated particles move faster, take up more space, and expand, even though their mass remains the same. This is because the density of a substance is determined by its mass and volume; when the volume increases while the mass stays constant, the density decreases.
- When a liquid or gas is heated, the particles at the bottom become less dense and rise through the cooler, denser parts of the substance, creating a convection current. This is why substances with lower density float on top of those with higher density. As the warmer particles rise, they are replaced by cooler, denser particles that sink, and this process continues, warming the entire volume.
- For instance, when a heater warms the air in a room, the air becomes less dense and rises. As it rises, it cools down by transferring thermal energy and eventually sinks back down, creating a cycle that heats the entire room. Similarly, in a cooking pan heated from below, convection currents form as hot, less dense water rises to the top and cooler, denser water sinks to the bottom, ensuring that the entire contents are heated evenly.
- Convection is possible in liquids and gases because their particles can move freely, unlike in solids where the particles only vibrate in fixed positions. Additionally, convection cannot occur in a vacuum because there are no particles present to facilitate the process.
Radiation
- Radiation is the process of transferring thermal energy through electromagnetic waves. Unlike conduction and convection, which require matter for heat transfer, radiation can occur through empty space.
- All objects emit radiant energy based on their temperature, with hotter objects emitting more energy. This phenomenon explains how the sun heats the Earth by radiating energy through the vacuum of space.
Conduction, convention and radiation
- Radiation transfers thermal energy in the form of waves, which enables energy to move through a vacuum. This is different from conduction and convection, which require a medium to transfer heat.
- For example, the thermal energy from the Sun reaches Earth in approximately nine minutes through radiation across the vacuum of space.
- All objects emit thermal energy through radiation, and hotter objects emit more radiation than cooler ones.
- Hot objects not only emit radiation but also cool objects absorb this radiation from them.
- Radiation can pass through vacuums as well as through transparent solids, liquids, and gases.
Factors Affecting Radiation Absorption and Emission
- Colour and Texture: The colour and texture of an object influence its ability to emit or absorb radiation.
- Best Emitters and Absorbers: Dull, black surfaces with large areas are the most effective emitters and absorbers of radiation.
- Poor Emitters and Absorbers: Shiny, white, or silver surfaces with smaller areas tend to reflect radiation and are the least effective at absorbing it.
Examples:
- Penguins have black feathers that help them absorb radiation from their surroundings for warmth.
- In hot climates, buildings are often painted white to reflect radiation and stay cooler.
How a Room Heater Works
A room heater transfers thermal energy through three processes:
- Conduction: Thermal energy is conducted from hot water to metal, then from metal to air.
- Convection: Warm air rises, distributing heat throughout the room.
- Radiation: The metal surface of the heater emits radiation, contributing to the warming of the room.
Vacuum Flasks
- Function: Vacuum flasks are designed to minimize heat transfer through conduction and radiation.
- Silvered Glass Bottle: The inner bottle is made of glass and coated with a reflective layer of silver. This layer reflects radiation back to the liquid inside, helping to maintain its temperature.
- Vacuum Insulation: The space between the inner bottle and the outer casing is a vacuum. This vacuum prevents heat transfer by conduction, as there are no particles to conduct heat.
Double-Glazed Windows
- Structure: Double-glazed windows consist of two layers of glass with a gap in between filled with argon gas.
- Insulation: Argon gas has low thermal conductivity, which means it does not conduct heat well. This property provides insulation, reducing heat transfer between the inside and outside of the building.
Wall Insulation
- Purpose: Wall insulation is used to reduce heat transfer through walls, making buildings more energy-efficient.
- Foam Insulation: One common type of wall insulation is foam. Foam insulation works by trapping air in its cells, which reduces heat transfer by conduction.
- Prevention of Convection Currents: Wall insulation also helps to stop convection currents, which are currents of air that can carry heat. By preventing these currents, insulation further reduces heat loss or gain.
Car Windows with Silver Coverings
- Function: Car windows with silver coverings are designed to reflect solar radiation.
- Heat Reduction: By reflecting solar radiation, these windows help to keep the interior of the car cooler. This is especially beneficial in hot weather when solar radiation can significantly increase the temperature inside a car.
Cooling by Evaporation
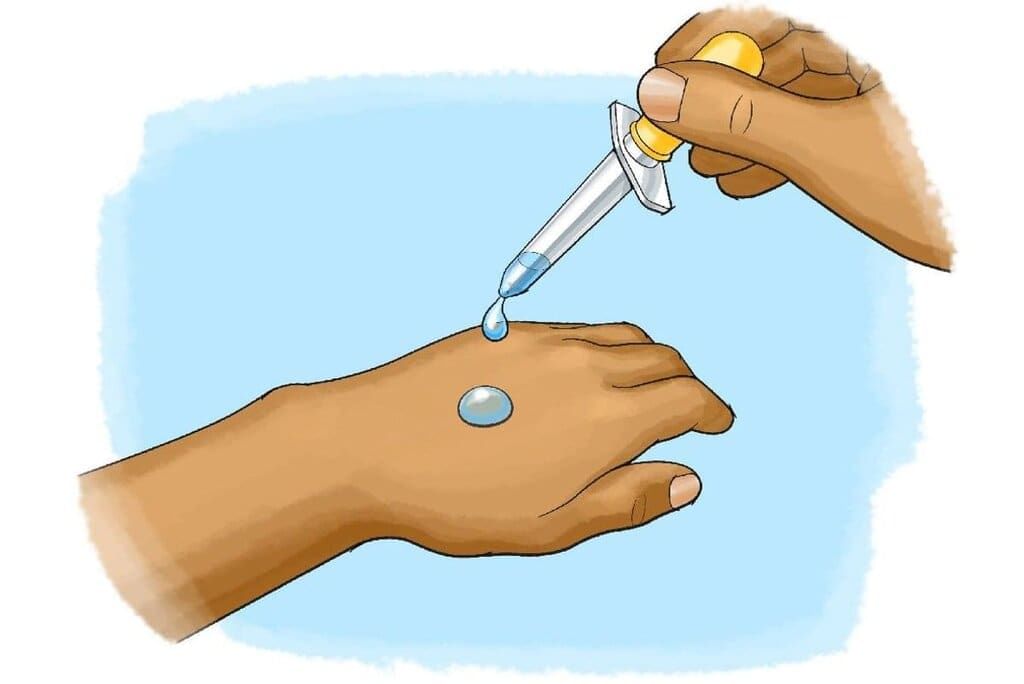
Evaporation
- Evaporation is the process where a liquid changes into a gas, and it plays a crucial role in cooling.
- In a liquid such as water at room temperature, the particles are in constant motion, but they move at different speeds. This random movement means that the particles are not all moving in the same direction or at the same pace.
Evaporation and Cooling
- The particles that are moving faster have more energy. Some of these particles have enough energy to break free from the surface of the liquid and escape into the air as gas particles.
- While some particles that try to escape are pulled back into the liquid, others manage to leave permanently. The particles that escape are usually the ones with the highest energy. The particles with medium energy might escape, but they could also be pulled back into the liquid, while the particles with the lowest energy remain in the liquid.
- Although the particles in a liquid are in contact with each other, not all of them are shown in diagrams for simplicity. This contact is important because it allows for the transfer of energy between particles, which is a key factor in the process of evaporation.
- Temperature refers to the average energy of particles in a substance. When high-energy particles escape from a liquid during evaporation , the average energy of the remaining particles decreases, leading to a reduction in the liquid’s temperature. This is why evaporation causes the remaining liquid to cool down.
- For instance, when water evaporates from the skin after swimming, it cools the skin by removing thermal energy, which is necessary for the liquid-to-gas transition. This process lowers the temperature of the skin.
Evaporation and Cooling of other liquid
- Sweat, which is produced at the temperature of the skin, also cools the skin when it evaporates. As sweat evaporates, its temperature decreases, allowing thermal energy to transfer from the skin to the cooler sweat, resulting in a cooling effect.
- Animals that do not sweat profusely rely on water evaporation for cooling, often by covering themselves with water.
- In humid conditions, the evaporation of sweat is hindered, which can lead to a dangerous rise in body temperature.
- For example, porous clay water coolers function by allowing water to seep through and evaporate, thereby cooling the water inside the container.
- Another instance is air coolers that utilize a water-soaked sponge; warm air blown through the sponge provides the thermal energy necessary for evaporation, resulting in cooler air.
- Different liquids possess varying strengths of inter-particle forces. Liquids with weaker forces, such as perfumes, evaporate more quickly, while those with stronger forces, like liquid soap, evaporate more slowly.
- Perfume, for example, evaporates faster than water, which removes thermal energy more rapidly and creates a colder sensation on the skin. Similarly, water evaporates faster than liquid soap, contributing to a cooler feeling due to the quicker removal of thermal energy.
- Safety: It is advisable to apply only water, soap, or perfume to the skin; soap should be rinsed off with ample water.
The document Forces and Energy Chapter Notes | Year 9 Science IGCSE (Cambridge) - Class 9 is a part of the Class 9 Course Year 9 Science IGCSE (Cambridge).
All you need of Class 9 at this link: Class 9
|
2 videos|38 docs|9 tests
|
FAQs on Forces and Energy Chapter Notes - Year 9 Science IGCSE (Cambridge) - Class 9
| 1. What is density and how is it calculated? |  |
Ans. Density is a measure of how much mass is contained in a given volume. It is calculated using the formula: Density = Mass/Volume. The units of density are often grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
| 2. How do heat and temperature differ from each other? |  |
Ans. Heat refers to the energy transferred between systems or objects due to a temperature difference, while temperature is a measure of the average kinetic energy of the particles in a substance. Essentially, heat is the energy in transit, and temperature is a measure of that energy within an object.
| 3. What are the three main ways to transfer thermal energy? |  |
Ans. The three main ways to transfer thermal energy are conduction, convection, and radiation. Conduction involves direct contact between materials, convection occurs in fluids where warmer areas rise and cooler areas sink, and radiation involves the transfer of energy through electromagnetic waves without needing a medium.
| 4. Why is understanding density important in various scientific fields? |  |
Ans. Understanding density is crucial in fields such as chemistry, physics, and engineering because it helps determine whether an object will float or sink in a fluid, influences material selection for construction, and is essential in processes such as buoyancy calculations and the design of various products.
| 5. How does thermal energy transfer affect everyday life? |  |
Ans. Thermal energy transfer affects everyday life in numerous ways, such as how we heat our homes (using convection), cook food (conduction), and even how we stay warm by absorbing sunlight (radiation). Understanding these principles helps improve energy efficiency and comfort in our daily activities.
Related Searches




















