NEET Exam > NEET Notes > Chemistry Class 12 > Revision Notes: Principles Related to Practical Chemistry
Revision Notes: Principles Related to Practical Chemistry | Chemistry Class 12 - NEET PDF Download
Detection of Extra Elements in Organic Compounds
Extra Elements Detected:
- Nitrogen: Detected using Lassaigne's test. The compound is fused with sodium metal in a fusion tube, and the resulting sodium extract is tested for the presence of ammonium ions (NH₄⁺) using Nessler's reagent.
Test Reaction: Sodium fusion extract + Nessler's reagent → Brown precipitate (Indicates presence of Nitrogen). - Sulphur: Detected by Lassaigne's test. The sodium extract is acidified and treated with lead acetate solution. A black precipitate of lead sulphide (PbS) confirms the presence of sulphur.
Test Reaction: Sodium fusion extract + Lead acetate → Black precipitate (PbS, confirms Sulphur). - Halogens: Halogens (Chlorine, Bromine, Iodine) are detected using Lassaigne's test. The sodium extract is acidified with dilute nitric acid and treated with silver nitrate solution. A white, yellow, or cream precipitate confirms chlorine, iodine, or bromine, respectively.
Test Reaction (Chlorine): Sodium fusion extract + Silver nitrate → White precipitate (AgCl, confirms Chlorine).
Detection of Functional Groups in Organic Compounds
1. Hydroxyl Group (Alcoholic and Phenolic):
- Alcoholic: Ferric chloride test gives a red coloration with phenols.
Reaction: Phenol + Ferric chloride → Red color (Indicates Phenol). - Phenolic: Sodium hydroxide test gives a bright yellow solution with phenols.
Reaction: Phenol + NaOH → Yellow color (Indicates Phenol).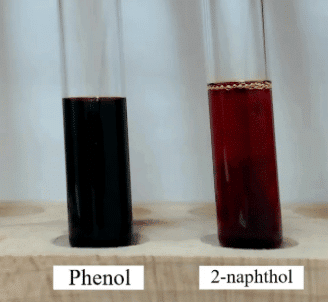
2. Carbonyl Group (Aldehyde and Ketones):
- Aldehyde: Tollens' Test (silver mirror test) and Fehling’s Test give a red precipitate of copper(I) oxide for aldehydes.
Reaction: Aldehyde + Tollens’ reagent → Silver mirror (Indicates Aldehyde).
Reaction: Aldehyde + Fehling’s solution → Red precipitate (Cu₂O). - Ketones: No reaction with Tollens' or Fehling’s solution.
3. Carboxyl Group:
- Carboxyl group is detected by adding sodium bicarbonate. Effervescence (release of CO₂) indicates the presence of a carboxyl group.
Reaction: R-COOH + NaHCO₃ → CO₂ effervescence (Indicates Carboxyl group).
4. Amino Group:
- Ninhydrin test is used for amino acids. A blue or purple color indicates the presence of an amino group.
Reaction: Amino acid + Ninhydrin → Blue/Purple color (Indicates Amino group). - Reaction with acetic acid gives a characteristic red color.
Reaction: Amino group + Acetic acid → Red color.
Preparation of Inorganic Compounds
- Mohr’s Salt (Ammonium Iron (II) Sulphate): Prepared by reacting ferrous sulphate (FeSO₄) with ammonium sulphate (NH₄)₂SO₄ in the presence of excess dilute sulfuric acid.
Reaction: FeSO₄ + (NH₄)₂SO₄ → (NH₄)₂Fe(SO₄)₂ + H₂O - Potash Alum (Potassium Aluminum Sulfate): Prepared by reacting potassium chloride (KCl) with aluminum sulfate (Al₂(SO₄)₃) in the presence of water.Reaction: Al₂(SO₄)₃ + 2 KCl → 2 KAl(SO₄)₂·12H₂O
Preparation of Organic Compounds
- Acetanilide: Prepared by reacting aniline with acetic anhydride.
Reaction: C₆H₅NH₂ + (CH₃CO)₂O → C₆H₅NHCOCH₃ + CH₃COOH - p-Nitro Acetanilide: Prepared by nitrating acetanilide with concentrated nitric acid and sulfuric acid.
Reaction: C₆H₅NHCOCH₃ + HNO₃ → C₆H₄(NO₂)NHCOCH₃ - Aniline Yellow: Prepared by the reaction of aniline with nitrous acid.
Reaction: C₆H₅NH₂ + HONO → C₆H₅N = N - C₆H₅ (Aniline yellow). - Iodoform (CHI₃): Prepared by the reaction of alcohol or aldehyde with iodine in the presence of sodium hydroxide.
Reaction: RCH(OH) + I₂ + NaOH → CHI₃ + RCOONa + H₂O
Titrimetric Exercises
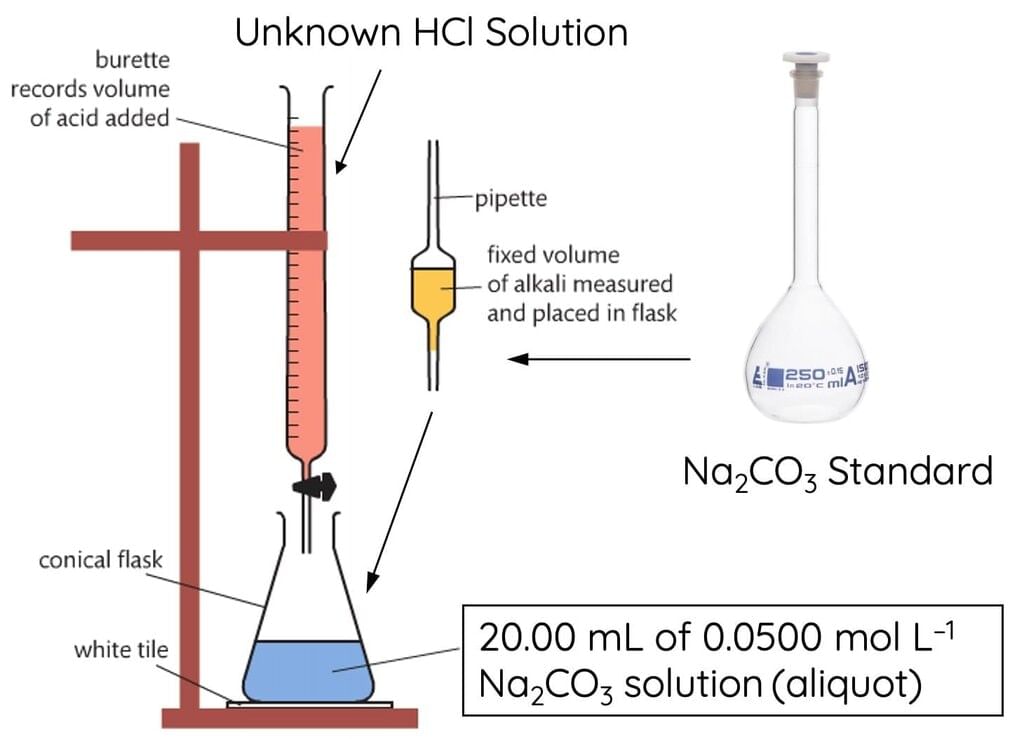
- Acids and Bases:
Strong Acid - Strong Base Titration: The pH indicator (such as phenolphthalein) is used to find the end point.
Reaction: HCl + NaOH → NaCl + H₂O - Oxalic Acid vs. KMnO₄: The reaction between oxalic acid (H₂C₂O₄) and KMnO₄ in acidic medium gives a color change.
Reaction: 5H₂C₂O₄ + 2KMnO₄ + 6H⁺ → 10CO₂ + 2Mn²⁺ + 8H₂O - Mohr’s Salt vs. KMnO₄: The reaction involves oxidation of ferrous ion (Fe²⁺) to ferric ion (Fe³⁺) by potassium permanganate (KMnO₄).
Reaction: 5Fe²⁺ + MnO₄⁻ + 8H⁺ → 5Fe³⁺ + Mn²⁺ + 4H₂O
Qualitative Salt Analysis
1. Cations:
- Pb²⁺ (Lead): Forms a yellow precipitate with KI.
Reaction: Pb²⁺ + 2I⁻ → PbI₂ (yellow precipitate). - Cu²⁺ (Copper): Forms a blue precipitate with ammonia.
Reaction: Cu²⁺ + 4NH₃ → [Cu(NH₃)₄]²⁺ (blue solution). - Fe³⁺ (Iron): Forms a red-brown precipitate with KSCN.
Reaction: Fe³⁺ + SCN⁻ → Fe(SCN)₃ (red-brown precipitate). - Zn²⁺ (Zinc): Forms a white precipitate with ammonia.
Reaction: Zn²⁺ + 2NH₃ + 2H₂O → [Zn(NH₃)₂(H₂O)₂]²⁺ (white precipitate). - Ca²⁺ (Calcium): Forms a white precipitate with ammonium oxalate.
Reaction: Ca²⁺ + C₂O₄²⁻ → CaC₂O₄ (white precipitate).
2. Anions:
- CO₃²⁻ (Carbonate): Reacts with HCl to release CO₂ gas.
Reaction: CO₃²⁻ + 2H⁺ → CO₂ + H₂O (effervescence). - S²⁻ (Sulfide): Forms a black precipitate of PbS with lead acetate.
Reaction: S²⁻ + Pb²⁺ → PbS (black precipitate). - SO₄²⁻ (Sulfate): Forms a white precipitate of BaSO₄ with BaCl₂.
Reaction: SO₄²⁻ + Ba²⁺ → BaSO₄ (white precipitate). - NO₃⁻ (Nitrate): Reduced to NO₂ (brown fumes) by sulfuric acid.
Reaction: 2NO₃⁻ + 10H⁺ + 8e⁻ → 2NO₂ + 4H₂O. - Cl⁻ (Chloride): Forms a white precipitate of AgCl with AgNO₃.
Reaction: Cl⁻ + Ag⁺ → AgCl (white precipitate).
Chemical Principles Involved in Experiments
- Enthalpy of Solution of CuSO₄: When CuSO₄ dissolves in water, the heat change is observed, which is the enthalpy change.
- Enthalpy of Neutralization of Strong Acid and Strong Base: The reaction between a strong acid (e.g., HCl) and a strong base (e.g., NaOH) produces water and releases heat, which is measured as enthalpy change.
Reaction: HCl + NaOH → NaCl + H₂O. - Preparation of Lyophilic and Lyophobic Sols:
- Lyophilic sols: Prepared by dissolving substances like gelatin in water, forming stable sols.
- Lyophobic sols: Prepared by dispersing metals like gold in a suitable liquid.
- Kinetic Study of the Reaction of Iodide Ions with Hydrogen Peroxide: The reaction follows the rate law and can be studied using the change in concentration of the reactants over time:
Reaction: H₂O₂ + 2I⁻ → I₂ + 2OH⁻
The document Revision Notes: Principles Related to Practical Chemistry | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|278 docs|78 tests
|
FAQs on Revision Notes: Principles Related to Practical Chemistry - Chemistry Class 12 - NEET
| 1. What are the common methods for detecting functional groups in organic compounds? |  |
Ans. Common methods for detecting functional groups in organic compounds include the use of chemical tests such as the bromine test for alkenes, the sodium bicarbonate test for carboxylic acids, and the 2,4-dinitrophenylhydrazine test for aldehydes and ketones. Additionally, spectroscopic methods like IR spectroscopy and NMR spectroscopy can provide information about functional groups based on characteristic absorption peaks and chemical shifts.
| 2. How can one prepare inorganic compounds in the laboratory? |  |
Ans. Inorganic compounds can be prepared through various methods, including precipitation reactions where two soluble salts react to form an insoluble compound, synthesis reactions that involve direct combination of elements, and thermal decomposition of metal carbonates or hydroxides. Each method requires careful control of reaction conditions and appropriate safety measures.
| 3. What is the importance of titrimetry in practical chemistry? |  |
Ans. Titrimetry is essential in practical chemistry as it allows for the precise determination of the concentration of a solute in a solution. This quantitative analytical technique involves reacting a solution of known concentration (titrant) with a solution of unknown concentration until the reaction reaches completion, indicated by a color change or other measurable endpoint. It is widely used in acid-base, redox, and complexometric titrations.
| 4. What is qualitative salt analysis and why is it important? |  |
Ans. Qualitative salt analysis involves the systematic identification of the cations and anions present in a salt sample. This process is crucial in chemistry as it helps in understanding the composition of unknown samples, determining the purity of compounds, and is vital in fields like pharmaceuticals, environmental testing, and forensic science. It typically involves a series of tests that lead to the identification of various ions based on their chemical behavior.
| 5. What chemical principles should be revised for practical chemistry in NEET preparation? |  |
Ans. Key chemical principles to revise for practical chemistry in NEET include stoichiometry, acid-base reactions, redox reactions, solubility rules, and the principles of chemical equilibrium. Understanding these concepts helps in accurately performing experiments, interpreting results, and solving problems related to quantitative and qualitative analysis effectively. Familiarity with laboratory techniques and safety protocols is also essential.
Related Searches
















